The outflow tract (OT) regions of the ventricles are a common location of origin for idiopathic ventricular arrhythmias (VA). Non-contact mapping (NCM) with a multi-electrode balloon catheter Ensite-Array enables three-dimensional reconstruction of the geometry of the cardiac chambers and accurate mapping of the propagation map, based on a single beat analysis, facilitating the ablation and contributing procedure success.
ObjectiveAssessment of the feasibility and long-term outcomes following NCM-guided OT VA ablation.
MethodsSingle center retrospective analysis of patients admitted for symptomatic OT VA ablation. Demographic and clinical characteristics of patients, procedure data and long-term outcomes were assessed.
ResultsFifty-eight patients (79.3% female, age 43.9±17.6 years) were considered, 89.7% without structural heart disease. In 85.7% of the cases left ventricle ejection fraction (LVEF) was preserved (LVEF≥50%), 8.6% had mild systolic dysfunction (LVEF 40%-49%) and 5.7% had moderate systolic dysfunction (LVEF 30%-39%). Twenty-four-hour Holter recording documented sustained VA episodes in 12.1% of the patients, non-sustained VA in 31.0%, and >10 000 premature ventricular complex (PVC)/24 h in 56.9%, with an ECG suggesting right ventricular OT origin in 84.5%. There was total elimination of PVC in 87.9% cases and a significant reduction in 3.4%. During a mean follow-up of 5.5 years, 87.9% patients remained asymptomatic without medication, 12.1% underwent re-ablation due to symptomatic PVC recurrence, and two cases underwent a third successful intervention.
ConclusionNon-contact mapping-guided multi-electrode balloon catheter VA ablation is a highly effective and safe procedure, with a low rate of long-term recurrence.
As câmaras de saída (CS) ventriculares são uma localização comum de origem de arritmias ventriculares (AV). O mapeamento não contacto (MNC) por balão multi-elétrodos Ensite-Array permite uma reconstrução geométrica tridimensional das câmaras cardíacas e um mapeamento preciso do mapa de propagação, baseado na análise de um único batimento, facilitando o procedimento de ablação e contribuindo para o seu sucesso.
ObjetivoAvaliação da viabilidade e outcomes em longo prazo após ablação de AV da CS guiada por MNC.
MétodosAnálise retrospetiva de pacientes admitidos num único centro para ablação de AV da CS sintomáticas. As caraterísticas demográficas e clínicas dos pacientes, dados do procedimento e outcomes em longo prazo foram avaliados.
ResultadosForam considerados 58 pacientes (79,3% mulheres, idade 43,9±17,6 anos) foram considerados, 89,7% sem doença cardíaca estrutural. Em 85,7% dos casos a fração de ejeção ventricular esquerda estava preservada (FEVE ≥ 50%), 8,6% tinham disfunção sistólica ligeira (FEVE 40%-49%) e 5,7% tinham disfunção sistólica moderada (FEVE 30%-39%). O Holter 24-horas documentou episódios de AV mantidas em 12,1% dos pacientes, AV não mantidas em 31,0% e >10.000 complexos ectópicos ventriculares (CEV)/24 h em 56,9%, com um ECG sugerindo uma origem na CS ventricular direita em 84,5%. Houve uma total eliminação dos CEV em 87,9% dos casos e uma redução significativa em 3,4%. Durante um follow-up médio de 5,5 anos, 87,9% dos pacientes permaneceram assintomáticos sem medicação, 12,2% foram submetidos a reablação devido a recorrência sintomática de CEV e dois casos fizeram uma terceira intervenção bem-sucedida.
ConclusãoA ablação de AV baseada no mapeamento não contacto por balão multi-elétrodos é um procedimento altamente eficaz e seguro, com uma baixa taxa de recorrência em longo prazo.
Premature ventricular complexes (PVC) are common arrythmias in clinical practice, occurring in 75% of the cases in the absence of structural heart disease, most of them originating from the right ventricular OT (RVOT).1–3 Triggered activity or abnormal automaticity, usually from a focal source, are the mechanisms underlying these arrhythmias.4 Many patients present with symptoms (mainly palpitations, but also chest pain and pre-syncope/syncope) associated with exercise, stress or the use of stimulants.1,3 Although symptoms can affect quality of life, idiopathic VA tends to have a benign prognosis. However, a high VA burden can result in left ventricular (LV) systolic dysfunction and cardiomyopathy and, rarely, OT VA can trigger idiopathic ventricular fibrillation.4,5 PVC may also derive from subclinical cardiomyopathy. Sometimes it is even impossible to determine whether the PVC or the cardiomyopathy is the cause or the consequence.2 The standard therapy to provide symptom relief is antiarrhythmic drugs. Beta blockers or verapamil usually show limited effectiveness.1,3,5 Other antiarrhythmic drugs, such as propafenone, flecainide, and specially amiodarone, have better outcomes, but are associated with adverse side effects.6 Several studies have demonstrated that radiofrequency (RF) ablation is more efficient than pharmacological therapy for eliminating PVC, especially for refractory symptoms and very frequent PVC.1–3,5 The number of patients being referred for ablation of OT VA continues to increase, especially earlier in their disease course, due to its efficacy (ranging between 83% and 100%) and safety (overall complication rate: 3.1-5.2%).2,7 However, RF ablation may be of limited use if PVCs are scarce, even with adrenergic and electrical stimulation in the electrophysiology laboratory, since activation mapping becomes more difficult. Also, pacemapping has a low spatial resolution.3,5,8 Noncontact mapping permits ablation guided by a single PVC analysis, which may facilitate ablation of these difficult cases.8 Due to technical challenges, including difficulty in handling and stabilizing the catheter inside the heart chambers, the non-contact balloon system has never been widely used.
In this study we describe our center's experience using the Ensite Array NCM system (St. Jude Medical, St. Paul, Minnesota, United States) for ablation of OT VA. To localize the focal arrythmia, this system uses a multielectrode array catheter with a 7.5 ml balloon and 64 microelectrodes. It is a method that can record endocardial electrograms from more than 3000 sites simultaneously from one single premature beat, to create a three-dimensional map of electrical activation.5,8–10
Material and methodsStudy populationThis is a retrospective cohort study of a tertiary single center population admitted for symptomatic OT VA ablation between 2000–2019. Inclusion criteria were monomorphic PVC>10 000/24 h or monomorphic VT, with left or right bundle branch block morphology on 12-lead electrocardiogram (ECG) (Figure 1) with suspected origin in the right or left ventricle OT, scheduled for catheter ablation due to frequent recurrent symptoms, refractoriness or intolerance of antiarrhythmic drugs, or patient preference. Baseline patient demographic characteristics, cardiovascular risk factors and associated comorbidities were recorded. Electrocardiogram or 24-h Holter QRS morphology, procedure data including the site of ablation and acute and long-term outcomes, were assessed.
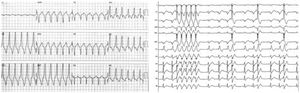
Electrocardiograms of two patients showing sustained monomorphic ventricular tachycardia (left image) and repetitive non-sustained monomorphic ventricular tachycardia (right image). Note the electrocardiographic morphology of left bundle branch block and inferior axis (left panel) and the rapid transition of QRS morphology from V1 to V2 (right panel).
All patients provided written informed consent both for the ablation procedure and for inclusion of their anonymized medical information into research studies. The latter was approved by the hospital's ethics committee. All ethical requirements present in the Helsinki Declaration of 1975 were met.
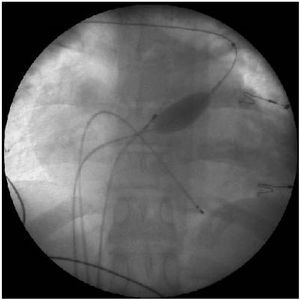
Electrophysiological study and ablationElectrophysiologic study and ablation procedure were performed in patients after six hours of fasting and after having been mildly sedated with midazolam. All antiarrhythmic drugs were suspended for at least five half-lives before the procedure. Non-contact mapping and ablation were performed with a multi-electrode balloon catheter Ensite Array positioned at the right or the left ventricle according to the suspected VA origin. All the procedures were performed by experienced operators. The NCM was introduced via the right femoral vein through a 10 Fr introducer and under fluoroscopy the balloon was placed in the ventricle OT 2-3 cm below the pulmonary valve or below the aortic valve through retrograde approach. Patients were heparinized to achieve an activated clotting time target around 300 seconds. Once positioned in the OT, the balloon was inflated and filled with iodinated contrast for radiological visualization (Figure 2). Subsequently, the three-dimensional geometry of the ventricle was acquired using the ablation catheter, with particular attention to the OT and the position of the semilunar valves.
In all patients, 12-lead ECG of any PVC, NSVT or VT were reviewed to ensure morphology identical to the clinical arrhythmia. An activation map was created based on recorded balloon catheter unipolar electrograms from spontaneous PVC that matched the clinical ectopies. This process was repeated at least 3 times to confirm the reproducibility of the PVC map. The criteria used to guide RF application was the early endocardial activation points during spontaneous PVC activity. The earliest activation point was defined as the point showing earliest activation with respect to QRS onset and a unipolar electrogram with a negative polarity and a fast downstroke. RF energy was applied with maximum temperature control of 40°C, energy power of 30W and maximum duration 60 seconds. In the absence of spontaneous VA, atrial and/or ventricular stimulation combined with isoproterenol administration was used to induce PVC.
Ablation strategy, success and follow-upVentricular arrhythmia (VA) morphology was categorized as “right bundle branch block” pattern if R/S ratio in lead V1 was ≥1 and as “left bundle branch block” pattern when R/S ratio in lead V1 was <1. For patients presenting with >1 VA morphologies, we considered the clinical VA to be that documented on a pre-ablation 12-lead ECG. RVOT was mapped first in patients presenting with “left bundle branch block” pattern VA, and left ventricle OT (LVOT) when VA had “right bundle branch block” or “left bundle branch block” with early QRS transition (before lead V3).
Acute success was defined as complete suppression of clinical VA, with no recurrence after a waiting period of 20 minutes. The occurrence of the arrhythmia after this period was considered recurrence. Failure in eliminating the arrhythmia was considered as acute procedure failure. Patients remained hospitalized until the next day with continuous ECG recording and a transthoracic echocardiography was performed after the intervention.
Patients were appraised in an outpatient setting four to eight weeks after ablation and subsequently at three-to-six-month intervals. 24 h-Holter was performed at four weeks and transthoracic echocardiography at six months and one year after the procedure. Long-term follow-up data were obtained by reviewing medical records and through a telephone interview with patients.
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics (IBM SPSS, Chicago, IL). Continuous variables were expressed as means ± standard deviation and categorical variables as absolute number and percentages.
ResultsClinical characteristics are presented in Table 1. The population comprised 58 patients, mean age 43.9±17.6 years, mostly female (79.3%), without any associated disease or comorbidity in 63.8% of the cases. Structural heart disease was excluded by echocardiography and/or cardiac magnetic resonance in 88% of the patients. In 85.7% of the patients left ventricle ejection fraction (LVEF) was preserved (LVEF≥50%), 8.6% had mild systolic dysfunction (LVEF 40-49%) and 5.7% had moderate systolic dysfunction (LVEF 30-39%).
Clinical characteristics of patients submitted to outflow tract ventricular arrhythmia ablation.
| Mean age (years) | 43.9±17.6 |
| Female gender (%) | 79.3 |
| Structural cardiopathy (N/%) | |
| Surgically corrected atrial septal defect Repaired | 1/1.7 |
| Fallot tetralogy | 1/1.7 |
| Ischemic cardiomyopathy | 1/1.7 |
| Idiopathic dilated cardiomyopathy | 1/1.7 |
| Mitral valve prolapse | 1/1.7 |
| Right ventricle dysfunction of unknown etiology | 2/3.5 |
| None | 51/88.0 |
| Associated comorbidities (N/%) | |
| Arterial hypertension | 14/24.1 |
| Dyslipidemia | 10/17.2 |
| Hypothyroidism | 1/1.7 |
| Asthma | 1/1.7 |
| Sarcoidosis | 1/1.7 |
| Ankylosing spondylitis | 1/1.7 |
| Bechet Disease | 1/1.7 |
| None | 37/63.8 |
| Symptoms (N/%) | |
| Palpitations | 43/74.1 |
| Fatigue | 8/13.8 |
| Pre-syncope/syncope | 7/12.1 |
| Ventricular arrhythmia (N/%) | |
| PVC | 33/56.9 |
| NSVA | 18/31.0 |
| SVA | 7/12.1 |
NSVA: non-sustained ventricular arrhythmias; PVC: premature ventricular complexes; SVA: sustained ventricular arrhythmias.
All patients were symptomatic. Most cases (74.1%) presented with palpitations, 13.8% with fatigue and 12.1% with presyncope/syncope. Twenty-four hour Holter recordings documented sustained VA episodes in 12.1%, non-sustained VA in 31.0%, and >10 000 PVC in 56.9%. The 12-lead ECG suggested an OT origin in all cases.
A large majority (77.6%) of patients were refractory to antiarrhythmic drugs: beta blockers 48.3%, amiodarone 8.6%, calcium channel antagonists 6.9%, flecainide 3.4% and sotalol 3.4%. In 7%, antiarrhythmic drugs were combined.
The origin of the arrhythmia was in the RVOT in 84.5% (n=49) and in the LVOT in 15.5% (n=9). The average number of RF energy deliveries to achieve success was 13±8. The earliest activation RVOT sites were: posteroseptal (31.5%), posterolateral (16.7%), posterior (13%), anterolateral (11.1%), and other locations (12.2%). In the LVOT, the earliest activation points were: anterior (5.3%), posterolateral (3.4%), posterolateral (3.4%), posterior (1.7%) and inferior (1.7%) (Figures 3 and 4).
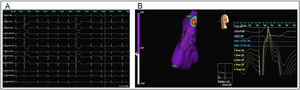
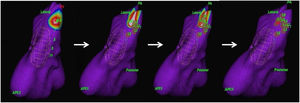
Three-dimensional electroanatomic mapping using the noncontact EnSite 3000. The acquisition of maps during sinus rhythm and ventricular premature complexes (PVC) identifies the early activation points. A: Surface twelve-lead ECG showing isolated monomorphic PVC originating from RVOT (left bundle branch block with an inferior axis pattern). B: Three-dimensional reconstruction of right ventricle outflow tract (left anterior oblique projection) and virtual electrograms at the site of earliest activation (note the QS pattern).
An example of successful ablation. Three-dimensional reconstruction of right ventricle anatomy (left anterior oblique projection) and identification of early activation points, marked in white/red (made possible by the acquisition of maps during sinus rhythm and premature ventricular complexes). Red dots (L1 to L9) indicate the sites of ablation therapy. The early activation point was seen to have moved away from the original site, where the first application was performed. Further ablation at the new site successfully abolished the ectopies.
Noncontact balloon guided ablation was acutely successful with total elimination of PVC in 87.9% patients (89.9% in RVOT origin and 100% in the LVOT cases). 3.4% patients achieved a >90% reduction of PVC. In 8.7% the ablation was non-successful. In 4 cases (6.9%), the procedure was complicated by small pericardial effusion (n=1), cardiac tamponade (n=2) and a pseudo-aneurysm of the femoral artery solved by ultrasound-guided compression (n=1). Peri-procedural complications were observed in patients with VA originating from the LVOT in three out of four cases; one patient with VA from the RVOT presented mild pericardial effusion.
Echocardiographic reevaluation between six months and one year after the procedure showed that 6.3% patients maintained mild systolic dysfunction, while 8% recovered systolic function to normal values. Patients with a previous preserved LVEF maintained within that range.
During a mean follow-up of 5.5 years, 87.9% of the patients achieved a reduction of >90% in total PVC burden on the Holter recordings and remained asymptomatic in the absence of antiarrhythmic drugs after the first ablation. 12.1% underwent re-ablation due to symptomatic PVC recurrence, and two cases underwent a third successful intervention.
DiscussionOur study confirms that OT VA in symptomatic patients or with very frequent PVC activity refractory to medical therapy can be safely eliminated based on tridimensional geometry and activation maps using NCM-guided multielectrode balloon catheter. NCM-based ablation was acutely successful in 87.9% of our population. Although mostly a young and healthy population, it reflected a challenging ablation group: 12% with structural cardiopathy and more than 80% with no sustained arrythmias to guide the electrophysiological mapping. Despite the fact that other authors have shown the usefulness of NCM-base ablatio in OT VA,6 its use is not widespread. In fact, the technical challenges and the long learning curve of this system, combined with the development of new catheters and mapping platforms from different manufacturers, have contributed to the recent discontinuation of the 64-pole NCM-guided Ensite Array Balloon. In our experience, acute success in mapping OT VA based on a single beat was high, with a low rate of recurrence during long-term follow-up.
Expert consensus statements on VA recommend catheter ablation for PVC in patients who remain symptomatic despite conservative treatment or who have probable reversible LV dysfunction.11,12 According to the recommendations, in our population, all patients were symptomatic before the first ablation procedure. The commonest symptom of OT arrhythmias is palpitations, also demonstrated in our study. In our experience, most patients remained asymptomatic after the first ablation, even in the absence of antiarrhythmic drugs. Lauck et al. demonstrated, through invasive continuous monitoring, a 50% reduction of cardiac output during ventricular bigeminism.13 The same reduction in cardiac output can be demonstrated with OT Doppler with transthoracic echocardiography, justifying the phenomenon of tachycardiomyopathy associated with frequent PVC, as demonstrated by Yarlagadda et al.14 Improvement in LVEF is other benefit after VA disappearance. In a meta-analysis performed by Lamba et al.,15 RF catheter ablation significantly improved LVEF by a mean of 10.36% (confidence interval 8.75-11.97, p<0.00001) in patients with frequent PVC originating from the RVOT.6 In our study, most patients had preserved LVEF prior to the ablation procedure. Of the 14.3% patients with LV systolic dysfunction, 8% recovered it to normal values after the ablation procedure.
In 8.7% of the cases, ablation was not successful. One of the cases could be attributed to the limitation imposed by the patient's anatomy, since it maintained an activation focus on an anterior position, not accessible to the ablation catheter due to the presence of the multielectrode balloon that was not possible to overcome. In the other cases, the failure was probably related to the difficulty with the substrate location, presumably with an intramyocardial or epicardial origin. These are considered the common mechanisms justifying procedure failure.8 Another aspect to be taken into account is that RF lesions usually have a diameter of 5 mm.16 The precise positioning of the ablation catheter in the arrhythmogenic tissue can be critical for the success of the ablation.8 Wen et al. described as factors associated with the failure of ablative therapy a delta wave-like QRS onset (suggesting epicardial origin) and short endocardial activation to QRS onset times, both suggesting distancing of the ablation catheter from the target.17 However, cases cataloged as immediate failure may not require re-ablation, since the simple modification of the automatic focus of ventricular ectopic activity seems to be sufficient to alter the refractoriness to antiarrhythmic therapy, was the case with a patient in our series. Although the procedure was acutely complicated in four cases due to pericardial effusion and a femoral pseudo-aneurysm, these conditions were all successfully resolved. Most importantly, our long-term success rate was high, with almost 90% of the patients remaining asymptomatic in the absence of antiarrhythmic drugs.
Study limitationsThe study's main limitation was the fact that it is a retrospective, single center, observational study. The reported results were not compared with conventional ablation techniques nor with the use of other three-dimensional mapping systems.
Despite the efficacy of this technique, there are some inherent limitations. The progression of the guidewire and the positioning of the inflated balloon in the OT can be difficult and may require a significant fluoroscopy time.6 Another limitation is when the distance between the central axis of the balloon catheter and the focus of the arrhythmia is greater than 4.0 cm. In this situation, the confidence of the activation map is impaired and there is a risk of applying RF in an inadequate site. This is particularly important in arrhythmias originating in sites other than the RVOT, which was the case in almost 15% of patients. Another limitation of the method is the contact of the balloon catheter with the endocardium in certain circumstances, especially when using isoproterenol, possibly generating ectopic activity different from the clinical one.6 All these limitations led to the discontinuation of this system more than one year ago. This is therefore can be considered as a “historical evaluation” since the 64-pole NCM-guided Ensite Array Balloon is no longer available on the market.
ConclusionNon-contact mapping-guided multi-electrode balloon catheter OT VA ablation in symptomatic patients or with very frequent PVC activity, refractory to medical therapy, is a highly effective and safe procedure, with a low rate of long-term recurrence.
Conflicts of interestThe authors have no conflicts of interest to declare.