Three-dimensional (3D) speckle tracking echocardiography (3DSTE) is a novel method for assessment of left atrial (LA) volumes and function without geometrical assumptions. 3DSTE allows detailed assessment of LA features including volume measurements, strain assessments and calculation of LA ejection force (LAEF). LA strain and volume-based functional parameters originate from the same 3D dataset, but assessment of LAEF requires more data including measurement of mitral annular dimensions and Doppler-derived inflow velocities. The present study was designed to find correlations between LAEF and 3DSTE-derived LA volume-based functional properties and strain parameters in healthy subjects.
MethodsThe study population comprised 34 randomly selected healthy subjects (age 36.1±11.2 years, 15 men) in sinus rhythm, all of whom had undergone standard two-dimensional transthoracic Doppler echocardiographic study extended with 3DSTE.
ResultsMitral annulus diameter-based LAEF correlated with global LA peak circumferential (r=0.39, p=0.02), longitudinal (r=0.32, p=0.05) and area (r=0.43, p=0.01) strain, total atrial stroke volume (r=0.30, p=0.05) and total atrial emptying fraction (r=0.31, p=0.05) characterizing (systolic) LA reservoir function and global LA 3D strain at atrial contraction (r=−0.44, p=0.01) and active atrial emptying fraction (r=0.36, p=0.04) characterizing (diastolic) LA contraction function (booster pump phase).
ConclusionsComplex LA functional assessment can be provided by 3DSTE, including calculation of LAEF and volume-based and strain functional properties, with significant correlations between these parameters.
A ecocardiografia tridimensional (3D) com speckle tracking (E3DST) é um novo método para avaliação do volume e da função da aurícula esquerda (AE) sem pressupostos geométricos. A E3DST permite a avaliação detalhada das características da AE, incluindo as medições volumétricas, as avaliações da pressão e o cálculo da força de ejeção da AE (FEAE). A força da AE e os parâmetros funcionais baseados no volume são provenientes dos mesmos dados 3D, mas a avaliação da FEAE requer mais dados incluindo a medição das dimensões do anel mitral e das velocidades do fluxo derivado do Doppler. Este estudo foi concebido para encontrar correlações entre a FEAE e a E3DST derivadas das propriedades funcionais baseadas no volume da AE e nos parâmetros de pressão em indivíduos saudáveis.
MétodosEste estudo incluiu 34 indivíduos saudáveis selecionados aleatoriamente (36,1 ± 11 anos, 15 homens) em ritmo sinusal, submetidos a estudo ecocardiográfico Doppler bidimensional transtorácico padrão associado a E3DST.
ResultadosA FEAE no anel mitral baseada no diámetro correlacionada com o pico global da AE circunferencial (r = 0,39, p = 0,02), longitudinal (r = 0,32, p = 0,05) e área (r = 0,43, p = 0,01) força, volume arterial total de acidente vascular cerebral (r = 0,30, p = 0,05) e fração auricular total de esvaziamento (r = 0,31, p = 0,05) caracterizando a função de reservatório da AE (sistólica) e a força 3D da AE na contração auricular (r = 0,44, p = 0,01) e fração auricular de esvaziamento ativa (r = 0,36, p = 0,04) caracterizando a função da contração da AE (diastólica) (fase da bomba de reforço).
ConclusõesA E3DST podia providenciar a avaliação funcional complexa da AE incluindo o cálculo da FEAE e das propriedades funcionais baseadas no volume e na força com correlações significativas entre estes parâmetros.
Three-dimensional (3D) echocardiography coupled with speckle tracking capability is a novel approach that may become a powerful methodology for the assessment of left atrial (LA) volumes and function without geometrical assumptions.1,2 Despite basic differences, volumetric real-time 3D echocardiography (RT3DE) and strain-based 3D speckle tracking echocardiography (3DSTE) were found to be comparable, reproducible and interchangeable for quantification of LA dimensions and functional properties.3 It is well known that LA function in the cardiac cycle is a complex process that includes storing of pulmonary venous return during left ventricular (LV) contraction and isovolumetric relaxation in systole (reservoir function), transfer of blood passively into the LV in early diastole (conduit function), and active contraction at late diastole (booster pump function).4 In earlier studies 3DSTE was used for detailed assessment of all LA features including volume measurements,5–9 strain assessment7–11 and calculation of LA ejection force (LAEF).12 LA strain and volume-based functional parameters originate from the same 3D dataset, but assessment of LAEF requires more data including measurement of mitral annular dimensions and Doppler-derived inflow velocities. However, the relationship between these functional properties has never been assessed. Therefore, the present study was designed to find correlations between LAEF and LA volume-based functional properties and strain parameters in healthy subjects.
Patients and methodsPatient populationThe study group consisted of 34 healthy subjects (mean age: 36.1±11.2 years, 15 men) in sinus rhythm. None had known disease or any factor which could theoretically affect the results. Data on these subjects were taken from the MAGYAR-Healthy study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle tRacking echocardiography in Healthy subjects) with the aim of clarifying the diagnostic and prognostic significance of 3DSTE-derived volume, strain, rotation and dyssynchrony parameters in healthy volunteers. Informed consent was obtained from all subjects. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local human research ethics committee.
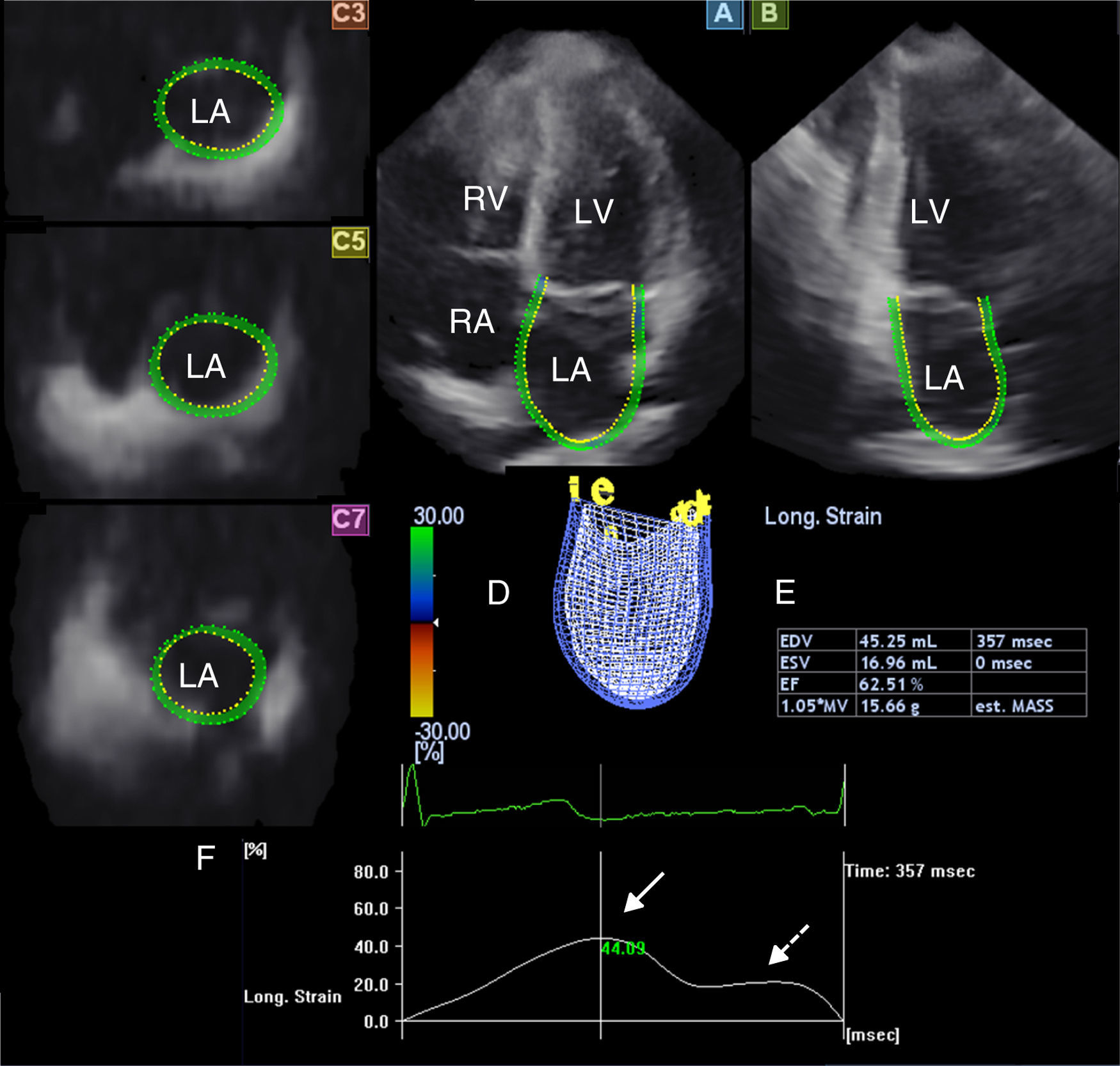
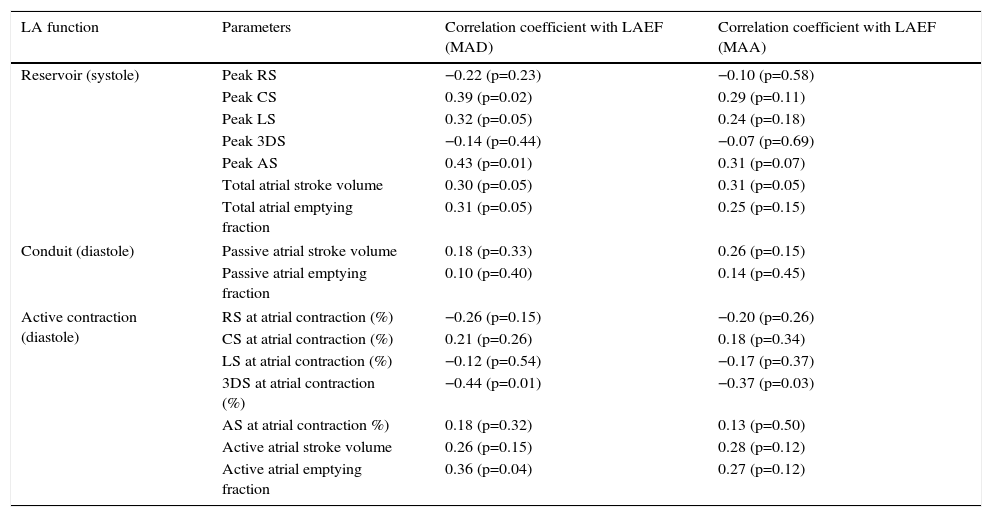
Three-dimensional speckle tracking echocardiographyAll 3DSTE studies were performed with a Toshiba Artida echocardiograph (Toshiba Medical Systems, Tokyo, Japan) using a 1–4 MHz PST-25SX matrix phased-array transducer.2 Acquisition of a full-volume 3D dataset required smaller wedge-shaped subvolumes from six consecutive cardiac cycles obtained during a single breathhold, which were then combined to provide the larger pyramidal 3D volume. LA quantification was performed using 3D Wall Motion Tracking software, version 2.7 (Toshiba Medical Systems, Tokyo, Japan). The 3D datasets were displayed in five different cross-sections comprising apical 2- (AP2CH) and 4-chamber (AP4CH) views and three standard short-axis views at different LA levels from the base to the apex. The orientation of the long axis of the AP2CH and AP4CH views was determined by positioning the main axis line to pass near the center of the LA cavity. The three short-axis views were defined by positioning the lines in AP2CH and AP4CH views at each level perpendicular to the LA long axis. The LA cavity was traced on the endocardium in AP2CH and AP4CH views starting at the edge of the septal mitral annulus (at the origin of the anterior mitral leaflet), then markers were placed in a counterclockwise rotation around the LA to the lateral mitral valve ring (to the origin of the posterior mitral leaflet). The LA epicardial border was manually adjusted. After detection of boundaries at the end-diastolic reference frame, wall motion tracking was then automatically performed through the entire cardiac cycle. A 3D cast together with volumetric and functional parameters of the LA were then generated (Figure 1).
The three-dimensional echocardiographic dataset is displayed in apical 4-chamber (A) and 2-chamber views (B) and three short-axis views in the basal (C3), mid-atrial (C5), and superior (C7) regions, respectively. Three-dimensional LA cast (D), LA volumetric data, ejection fraction (EF) (E) and global peak longitudinal strain (white arrow) and global longitudinal strain at atrial contraction (dashed arrow) (F) are also presented. LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle.
From the 3D model of the LA, maximum LA volume (at end-systole, largest LA volume before mitral valve opening [Vmax]), minimum LA volume (at end-diastole, smallest LA volume before mitral valve closure [Vmin]) and LA volume before atrial contraction (at time of P wave on ECG [VpreA]) were calculated (Figure 1).5–9 To characterize the reservoir, conduit and active contraction phases of LA function, stroke volumes (SV) and emptying fractions (EF) were measured from the three volumes using the following equations:
Reservoir function:
- -
Total atrial stroke volume (TASV): Vmax−Vmin
- -
Total atrial emptying fraction (TAEF): TASV/Vmax×100
Conduit function:
- -
Passive atrial stroke volume (PASV): Vmax−VpreA
- -
Passive atrial emptying fraction (PAEF): PASV/Vmax×100
Active contraction:
- -
Active atrial stroke volume (AASV): VpreA−Vmin
- -
Active atrial emptying fraction (AAEF): AASV/VpreA×100.
The main advantage of 3DSTE is that from the same 3D model of the LA, several functional parameters including strains can be easily measured.7–11 On the basis of one-directional radial, longitudinal and circumferential strains, area strain (ratio of endocardial area change during the cardiac cycle) and 3D strain (strain in the wall thickening direction, combination of one-directional strains) can also be calculated. Global peak strains (characterizing LA reservoir function) and global strains at atrial contraction (characterizing LA active contraction function) were measured for each subject (Figure 1).
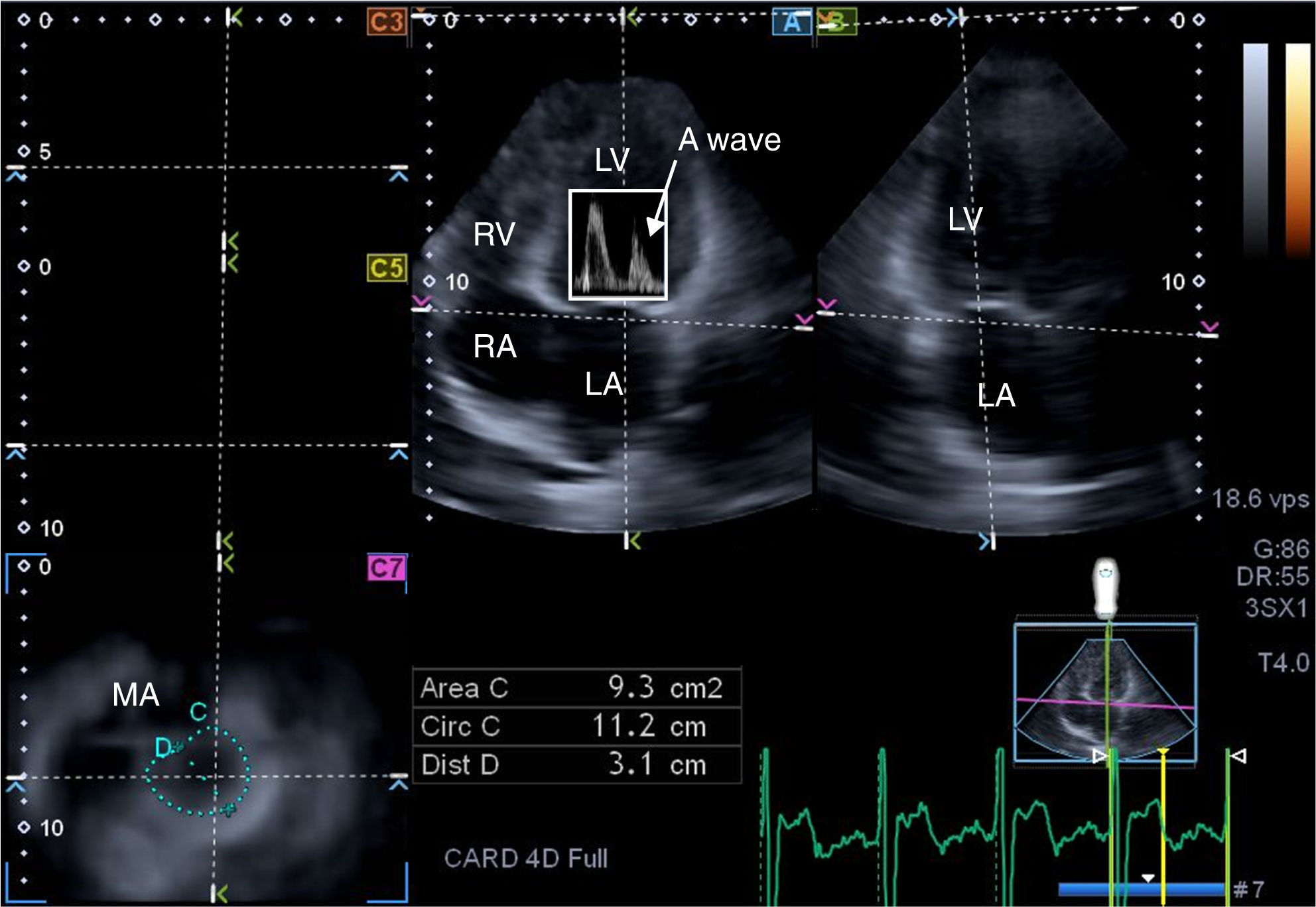
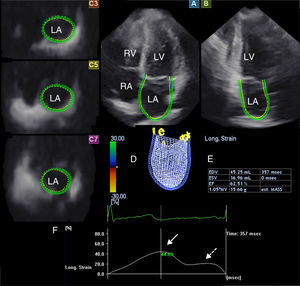
Three-dimensional speckle tracking echocardiography for left atrial ejection force measurementsThere is a third way to analyze LA function, by calculating LAEF. According to Newton's second law of motion, the force generated by the LA in its active contraction phase can be calculated using the following equation: LAEF=0.5×1.06×(MAD or MAA)×V2, where 0.5 is a coefficient, 1.06 g/cm3 is the blood density, MAD is the mitral annulus diameter, MAA is the mitral annulus area, and V is the peak A wave velocity.13 From the same 3D echocardiographic dataset, the mitral annulus (MA) can be obtained by optimizing cross-sectional planes on the endpoints of the MA in AP4CH and AP2CH views12 (Figure 2). MAD is then defined as the perpendicular line drawn from the top of the MA curvature to the middle of the straight MA border, while MAA can also be measured using planimetry. For measurement of LAEF, diastolic MAD and MAA data were used.
From the three-dimensional echocardiographic dataset, the mitral annulus (MA) can be obtained by optimizing cross-sectional planes in apical 4-chamber (A) and 2-chamber (B) views, demonstrating an optimal MA image on cross-sectional view (C7). Using Doppler-derived mitral inflow peak A wave velocity, the left atrial ejection force (LAEF) can be calculated. E and A: Doppler-derived mitral inflow velocities; LA: left atrium; LV: left ventricle; MA: mitral annulus; RA: right atrium; RV: right ventricle.
Continuous variables are expressed as mean values ± standard deviation, while categorical data are summarized as percentages. A p value of <0.05 was considered to indicate statistical significance. Pearson's coefficient was used for correlations. Recently, intra- and interobserver agreement for LA volumes and functional properties were assessed in papers based on the MAGYAR-Healthy and MAGYAR-Path studies.6,8 However, 3DSTE-derived MAD and MAA were not assessed. Intra- and interobserver agreements were studied according to Bland and Altman's method.14 All calculations were performed with commercially available software (MedCalc, Mariakerke, Belgium).
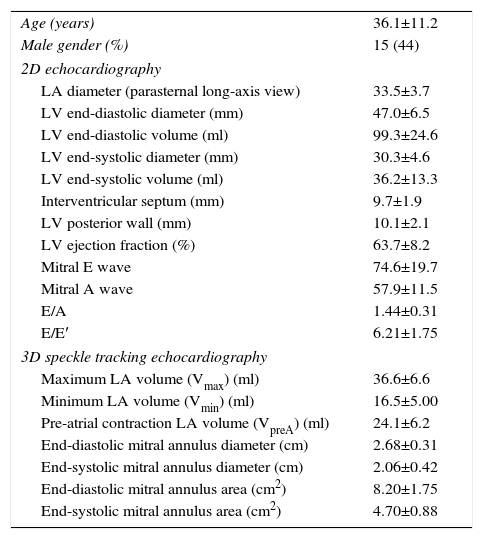
ResultsClinical and echocardiographic dataBaseline clinical and echocardiographic data for the study population are presented in Table 1. All two-dimensional (2D) echocardiographic and 3DSTE-derived data were in normal ranges in this healthy population.
Clinical, two-dimensional and three-dimensional speckle tracking echocardiographic data of the study population.
| Age (years) | 36.1±11.2 |
| Male gender (%) | 15 (44) |
| 2D echocardiography | |
| LA diameter (parasternal long-axis view) | 33.5±3.7 |
| LV end-diastolic diameter (mm) | 47.0±6.5 |
| LV end-diastolic volume (ml) | 99.3±24.6 |
| LV end-systolic diameter (mm) | 30.3±4.6 |
| LV end-systolic volume (ml) | 36.2±13.3 |
| Interventricular septum (mm) | 9.7±1.9 |
| LV posterior wall (mm) | 10.1±2.1 |
| LV ejection fraction (%) | 63.7±8.2 |
| Mitral E wave | 74.6±19.7 |
| Mitral A wave | 57.9±11.5 |
| E/A | 1.44±0.31 |
| E/E′ | 6.21±1.75 |
| 3D speckle tracking echocardiography | |
| Maximum LA volume (Vmax) (ml) | 36.6±6.6 |
| Minimum LA volume (Vmin) (ml) | 16.5±5.00 |
| Pre-atrial contraction LA volume (VpreA) (ml) | 24.1±6.2 |
| End-diastolic mitral annulus diameter (cm) | 2.68±0.31 |
| End-systolic mitral annulus diameter (cm) | 2.06±0.42 |
| End-diastolic mitral annulus area (cm2) | 8.20±1.75 |
| End-systolic mitral annulus area (cm2) | 4.70±0.88 |
2D: two-dimensional; 3D: three-dimensional; LA: left atrial; LV: left ventricular.
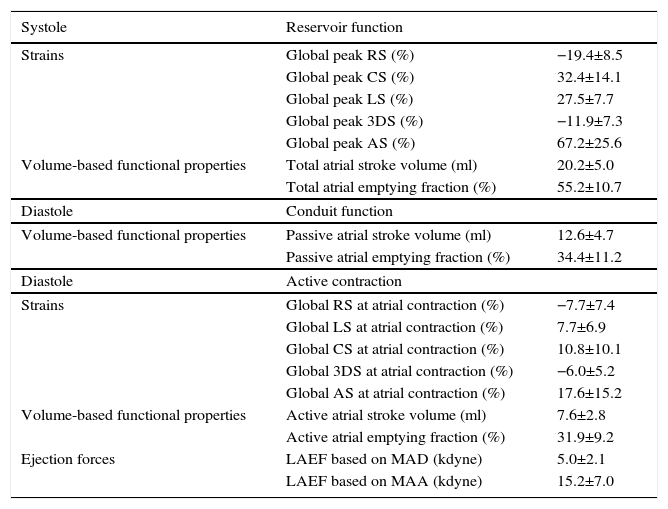
Volume-based and strain parameters derived from 3DSTE characterizing all phases of LA function together with LAEF are presented in Table 2.
Characteristics of left atrial function.
| Systole | Reservoir function | |
|---|---|---|
| Strains | Global peak RS (%) | −19.4±8.5 |
| Global peak CS (%) | 32.4±14.1 | |
| Global peak LS (%) | 27.5±7.7 | |
| Global peak 3DS (%) | −11.9±7.3 | |
| Global peak AS (%) | 67.2±25.6 | |
| Volume-based functional properties | Total atrial stroke volume (ml) | 20.2±5.0 |
| Total atrial emptying fraction (%) | 55.2±10.7 | |
| Diastole | Conduit function | |
| Volume-based functional properties | Passive atrial stroke volume (ml) | 12.6±4.7 |
| Passive atrial emptying fraction (%) | 34.4±11.2 | |
| Diastole | Active contraction | |
| Strains | Global RS at atrial contraction (%) | −7.7±7.4 |
| Global LS at atrial contraction (%) | 7.7±6.9 | |
| Global CS at atrial contraction (%) | 10.8±10.1 | |
| Global 3DS at atrial contraction (%) | −6.0±5.2 | |
| Global AS at atrial contraction (%) | 17.6±15.2 | |
| Volume-based functional properties | Active atrial stroke volume (ml) | 7.6±2.8 |
| Active atrial emptying fraction (%) | 31.9±9.2 | |
| Ejection forces | LAEF based on MAD (kdyne) | 5.0±2.1 |
| LAEF based on MAA (kdyne) | 15.2±7.0 |
3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; LAEF: left atrial ejection force; LS: longitudinal strain; MAA: mitral annular area; MAD: mitral annular diameter; RS: radial strain.
Both MAD- and MAA-based LAEF showed correlations with global 3D strain at atrial contraction, and MAD-based LAEF correlated with AAEF (Table 3). Although LAEF is a characteristic of LA booster pump function, correlations could be demonstrated between LAEF and volume-based and strain characteristics of LA reservoir function, as well global peak strains, TASV and TAEF. No correlation could be demonstrated between LAEF and parameters characterizing LA conduit function (PASV, PAEF).
Correlations between left atrial ejection force and other characteristics of left atrial function.
| LA function | Parameters | Correlation coefficient with LAEF (MAD) | Correlation coefficient with LAEF (MAA) |
|---|---|---|---|
| Reservoir (systole) | Peak RS | −0.22 (p=0.23) | −0.10 (p=0.58) |
| Peak CS | 0.39 (p=0.02) | 0.29 (p=0.11) | |
| Peak LS | 0.32 (p=0.05) | 0.24 (p=0.18) | |
| Peak 3DS | −0.14 (p=0.44) | −0.07 (p=0.69) | |
| Peak AS | 0.43 (p=0.01) | 0.31 (p=0.07) | |
| Total atrial stroke volume | 0.30 (p=0.05) | 0.31 (p=0.05) | |
| Total atrial emptying fraction | 0.31 (p=0.05) | 0.25 (p=0.15) | |
| Conduit (diastole) | Passive atrial stroke volume | 0.18 (p=0.33) | 0.26 (p=0.15) |
| Passive atrial emptying fraction | 0.10 (p=0.40) | 0.14 (p=0.45) | |
| Active contraction (diastole) | RS at atrial contraction (%) | −0.26 (p=0.15) | −0.20 (p=0.26) |
| CS at atrial contraction (%) | 0.21 (p=0.26) | 0.18 (p=0.34) | |
| LS at atrial contraction (%) | −0.12 (p=0.54) | −0.17 (p=0.37) | |
| 3DS at atrial contraction (%) | −0.44 (p=0.01) | −0.37 (p=0.03) | |
| AS at atrial contraction %) | 0.18 (p=0.32) | 0.13 (p=0.50) | |
| Active atrial stroke volume | 0.26 (p=0.15) | 0.28 (p=0.12) | |
| Active atrial emptying fraction | 0.36 (p=0.04) | 0.27 (p=0.12) | |
3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; LAEF: left atrial ejection force; LS: longitudinal strain; MAA: mitral annular area; MAD: mitral annular diameter; RS: radial strain.
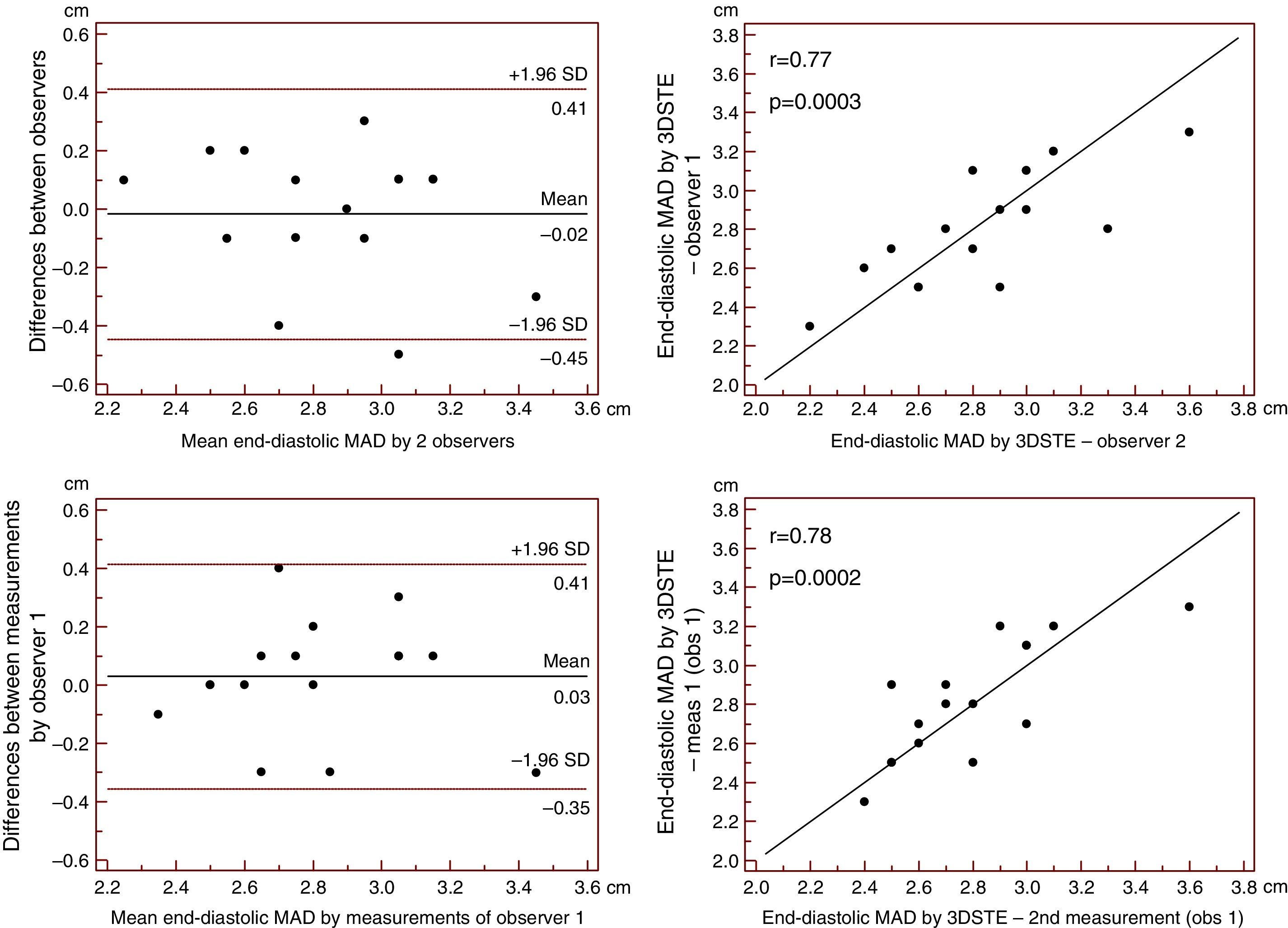
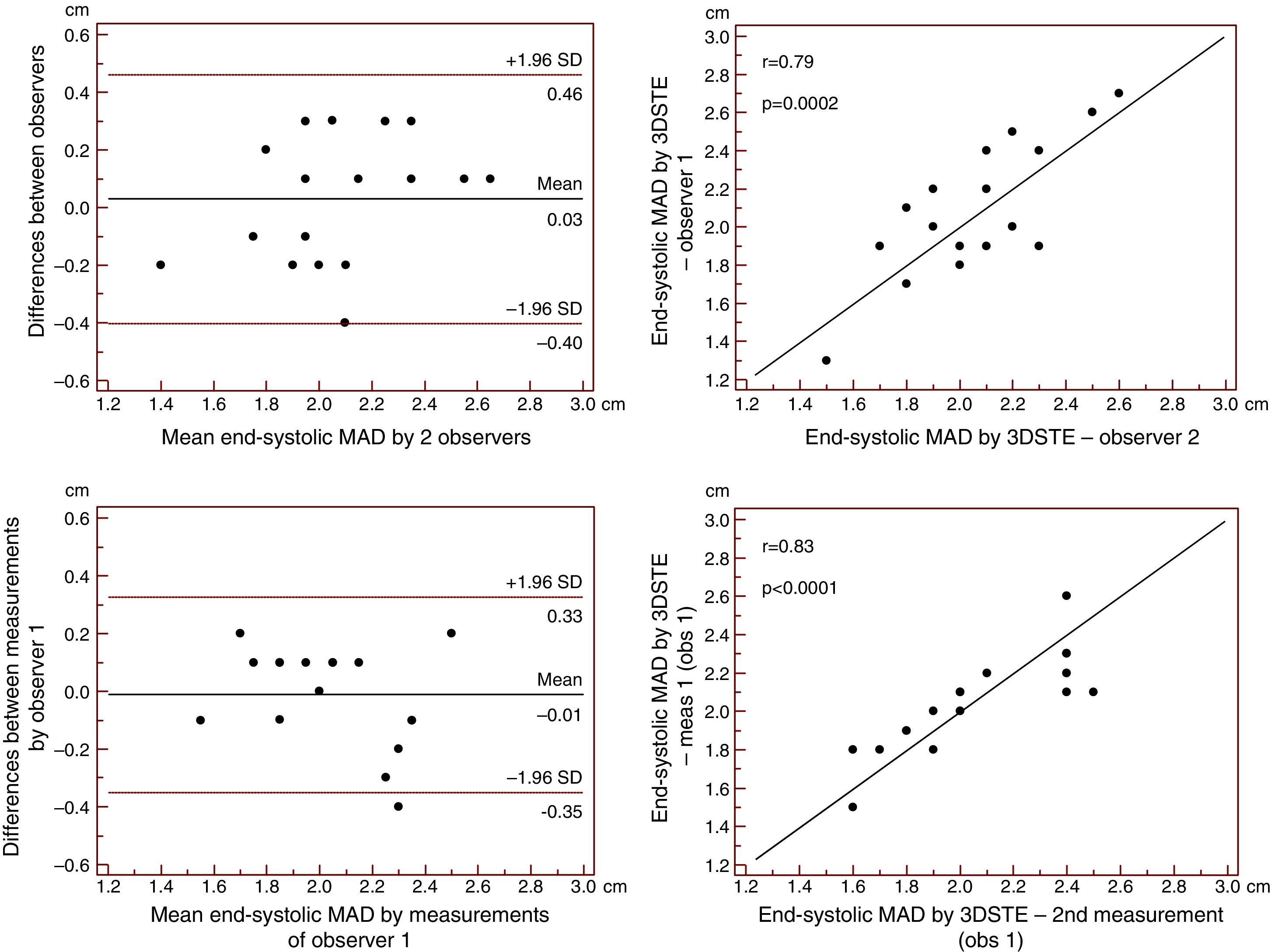
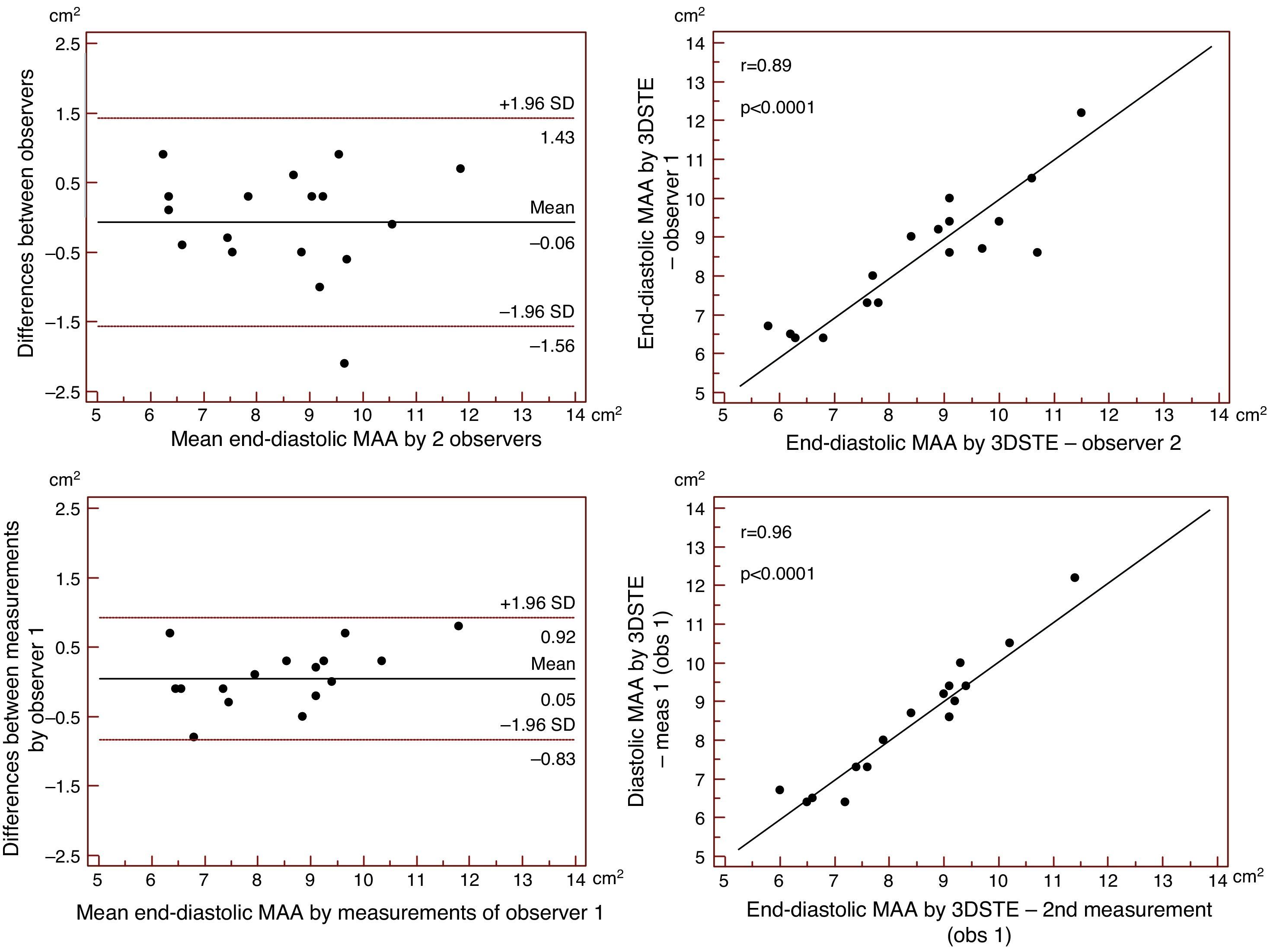
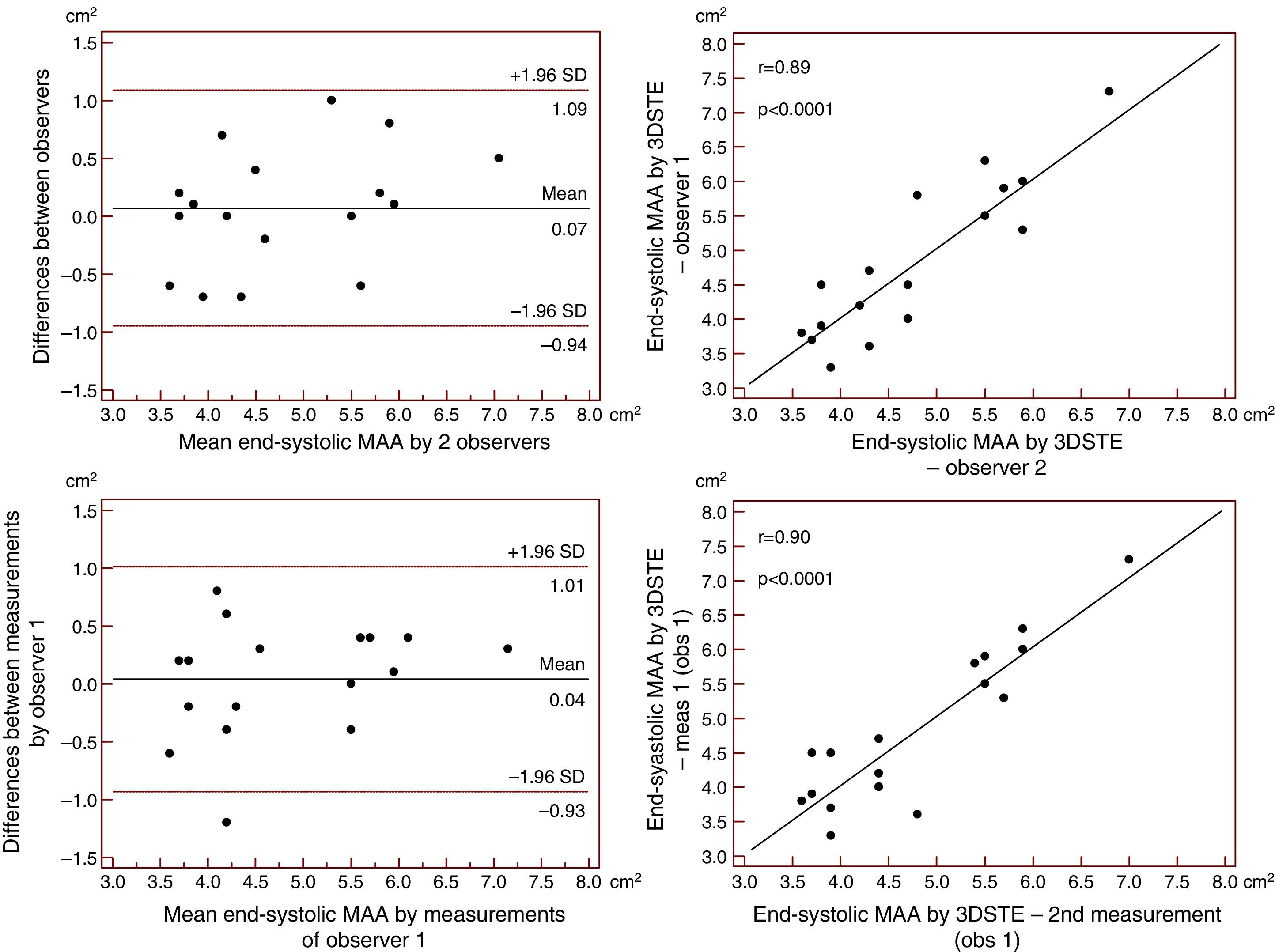
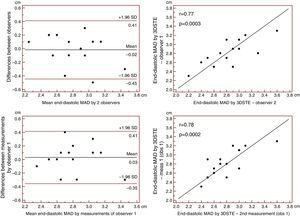
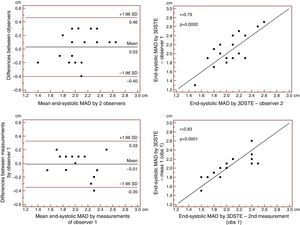
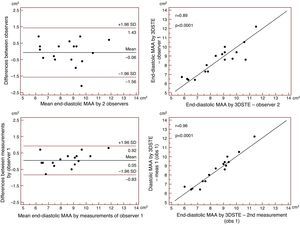
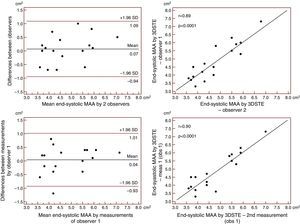
Reproducibility measurements were performed in 17 healthy controls. The mean ± standard deviation differences in values obtained by two observers for the measurements of 3DSTE-derived diastolic and systolic MAD and diastolic and systolic MAA were −0.02±0.43 cm, 0.02±0.43 cm, −0.06±1.49 cm2 and 0.07±1.02 cm2, respectively. Correlation coefficients between measurements of two observers were 0.77, 0.79, 0.89 and 0.89 (p=0.0003, 0.0002, <0.0001 and <0.0001), respectively (interobserver agreement) (Figures 2–5). The mean ± standard deviation differences in values obtained in two measurements by the same observer for 3DSTE-derived diastolic and systolic MAD and diastolic and systolic MAA were 0.03±0.38 cm, −0.01±0.34 cm, 0.05±0.87 cm2, and 0.04±0.97 cm2, respectively. Correlation coefficients between these independent measurements by the same observer were 0.78, 0.83, 0.96 and 0.90 (p=0.0002, <0.0001, <0.0001 and <0.0001), respectively (intraobserver agreement) (Figures 3–6).
Interobserver (upper graphs) and intraobserver (lower graphs) agreements and correlations for measuring end-diastolic mitral annulus diameter by three-dimensional speckle tracking echocardiography are presented. 3DSTE: three-dimensional speckle tracking echocardiography; MAD: 3DSTE-derived mitral annulus diameter; obs: observer.
Interobserver (upper graphs) and intraobserver (lower graphs) agreements and correlations for measuring end-systolic mitral annulus diameter by three-dimensional speckle tracking echocardiography are presented. 3DSTE: three-dimensional speckle tracking echocardiography; MAD: 3DSTE-derived mitral annulus diameter; obs: observer.
Interobserver (upper graphs) and intraobserver (lower graphs) agreements and correlations for measuring end-diastolic mitral annulus area by three-dimensional speckle tracking echocardiography. 3DSTE: three-dimensional speckle tracking echocardiography; MAA: 3DSTE-derived mitral annulus area; obs: observer.
Interobserver (upper graphs) and intraobserver (lower graphs) agreements and correlations for measuring end-systolic mitral annulus area by three-dimensional speckle tracking echocardiography. 3DSTE: three-dimensional speckle tracking echocardiography; MAA: 3DSTE-derived mitral annulus area; obs: observer.
The newly developed 3DSTE is a non-invasive imaging methodology with chamber quantification capability based on block-matching of the myocardial speckles of the endocardial border during their frame-to-frame motion.2 The usefulness of 3DSTE for LA volumetric assessments has been demonstrated and validated by 2D echocardiography,6 two-dimensional speckle tracking echocardiography (2DSTE),5 RT3DE3 and computed tomography.5 Moreover, 3DSTE-derived LA strain measurements have also been reported7–11 and validated by 2DSTE.10
In most cases, volumetric and strain assessments can be performed simultaneously using the same 3D model of the LA. However, there is a third way to characterize LA function during the same examination, by measuring LAEF, the force exerted by the LA to accelerate blood into the LV during atrial systole. LAEF is based on classic Newtonian mechanics and is a potentially useful index for assessing atrial contribution to diastolic performance.13 Compared to 2D imaging, both 3D echocardiographic techniques, RT3DE15–17 and 3DSTE,12 have been demonstrated to be practicable in assessing LAEF using Doppler-derived mitral inflow A velocity. However, to the best of the authors’ knowledge this is the first time possible correlations have been examined between 3DSTE-derived LAEF and LA strain and volume-based functional properties to assess cardiac systolic and diastolic function in healthy subjects.
In a recently published paper from the MAGYAR-Healthy Study, 3DSTE appeared to be feasible in detecting cyclic changes in LA volume, and calculation of its functional properties was comparable to 2D echocardiography. Good correlations were found between the two techniques for LA volumetric data and volume-based functional properties.6 Moreover, excellent intra- and interobserver agreement were demonstrated for 3DSTE-derived volumetric6 and strain data.6,8 The results of the present study extend our knowledge, demonstrating the ability of 3DSTE to reproducibly assess MAD and MAA and allowing simple calculation of LAEF.
The study reported here is the first to demonstrate correlations between LAEF and 3DSTE-derived volume-based and strain parameters featuring systolic LA reservoir and late diastolic LA booster pump phases calculated from the same 3D model of the LA. No relationships could be demonstrated between LAEF and functional properties of early diastole (LA conduit function). The results of the present study highlight several important points. Firstly, 3DSTE appears to be a simple, non-invasive technology that enables complex evaluation of LA function: all LA functions, including systolic reservoir, early diastolic conduit and late diastolic booster pump (or active contraction) phases, can be assessed at the same time in detail. Secondly, several volume-based and strain parameters can be calculated from the same 3D model of the LA. The measurement of LAEF requires more data including MAD or MAA and pulsed Doppler-derived mitral inflow A wave. Thirdly, significant correlations can be demonstrated between these functional properties, as demonstrated above. However, further validation studies with other imaging methodologies are warranted to confirm our findings. Moreover, other studies should focus on deeper insights into atrial (patho)physiology, especially in different cardiovascular disorders, using all the methodologies detailed above.
LimitationsIn agreement with the available literature, the LA appendage and pulmonary veins were excluded from evaluations. Although most patients had far from optimal image quality due to low temporal and spatial resolutions, none of them were excluded from the analyses, but could theoretically affect the results. Only a limited number of healthy volunteers from a single center were examined and the measurements were made by a single observer (DP).
ConclusionsComplex LA functional assessment can be provided by 3DSTE, including calculation of LAEF and volume-based and strain functional properties, with significant correlations between these parameters.
Conflicts of interestThe authors have no conflicts of interest to declare.