Poisoning by ingestion of ‘Jamaican Stone’, a kind of cardioactive steroid, is extremely rare. However, mortality is very high. For this reason, when it occurs, an early and accurate diagnosis represents a critical challenge for clinicians. We present an unusual case of electrical storm caused by this substance.
O envenenamento por ingestão de «pedra jamaicana», um tipo de esteroide cardioativo, é extremamente raro. Contudo, dado esta situação se associar a uma mortalidade muito elevada, o diagnóstico precoce e rigoroso desta situação pode ser clinicamente crucial. É retratado um caso pouco comum de tempestade arrítmica causada por esta substância.
‘Jamaican Stone’, also known ‘Love Stone’ or ‘Chan Su’, is a kind of cardioactive steroid (CAS) derived from toad venom. In western countries, poisoning caused by ingestion of this substance is very uncommon. Thus, when it occurs, an early and accurate diagnosis represents a critical challenge for clinicians.
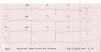
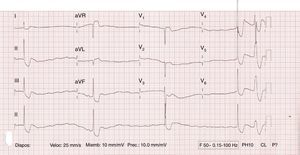
Case reportA previously healthy 32-year-old male presented with abdominal pain, nausea, weakness and vomiting after accidental ingestion of ‘Jamaican Stone’, a topical remedy applied direct to the penis for delaying ejaculation. On arrival at the hospital he was somnolent. Initial blood pressure was 85/30 mmHg and the electrocardiogram showed complete atrioventricular block with diffuse ST-segment depression (Figure 1). Treatment with dopamine was initiated. Transthoracic echocardiography revealed a hyperdynamic left ventricle and potassium was within normal limits.
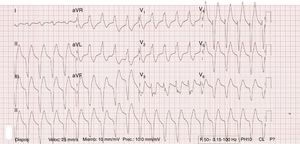
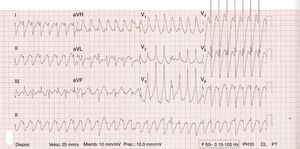
Five minutes later he developed a 130 bpm regular wide QRS tachycardia that subsequently progressed to ventricular fibrillation treated by a shock. After defibrillation, asystole was observed, and advanced cardiopulmonary resuscitation was performed. Multiple recurrences of ventricular arrhythmias (Figure 2), including bidirectional ventricular tachycardia (VT) (Figure 3), were recorded, followed by ventricular fibrillation. Likewise, transcutaneous pacing resulted in VT, and lidocaine infusion was therefore started. There was no recurrence of ventricular arrhythmias but he remained in complete atrioventricular block. A transvenous pacemaker was then successfully implanted.
Although intensive care treatment was performed, the patient's condition gradually worsened and he finally died 24 hours after ingestion.
DiscussionCASs consist of a steroid nucleus and an unsaturated 5-membered (cardenolide) or 6-membered (bufadienolide) lactone ring. Most cardenolides are derived from plants (such as Digitalis lanata, Nerium oleander or Thevetia peruviana). By contrast, bufadienolides are derived mainly from mammals and amphibians (such as toad venom, used to produce Jamaican Stone).
Cardenolides have been more often described in the literature.1 Digoxin, a well-known cardenolide, is commonly used to treat atrial fibrillation and congestive heart failure, and the clinical effects and symptoms of overdose are well recognized. Toxicity due to other CASs is similar to digoxin poisoning.2,3
Presentation as vomiting and abdominal pain is commonest, and neurological manifestations are also frequent in more severe cases. Bradyarrhythmias are the most common manifestation (conduction defects affecting the sinus node, the atrioventricular node or both), but tachyarrhythmias, atrial (usually atrial fibrillation) or ventricular, may also occur.
Clinical suspicion is the key, and positivity of serum immunoassays for digoxin (cross-reactivity with CASs) may corroborate diagnosis. Because of partial cross-reactivity, the concentration by itself is not relevant or predictive of clinical outcome.
Treatment in severe cases is very difficult and mortality associated with exposure to nonpharmaceutical CASs is high.
A systematic review of 924 cases of CAS poisoning by Barrueto et al. found overall mortality of 6.7%, and bufadienolides (3% of cases) were five times more lethal than cardenolides (29.6% vs. 6%, respectively).4
Prevention of gastrointestinal absorption of further toxin by emesis, gastric lavage, administration of activated charcoal and cathartics is intuitive, may be useful and is harmless.
When significant bradyarrhythmia is present, pacemaker therapy is recommended. Use of atropine is frequently reported in the literature, but opinion diverges about its potential to induce tachyarrhythmia. Catecholamines, although there are references to beneficial effects, are not advised because they may potentiate ventricular arrhythmias, as the electrophysiologic effects underlying CAS toxicity seem to be related to delayed afterdepolarizations (DADs) and triggered arrhythmias secondary to Ca2+ overload.
Serum potassium concentration is frequently altered and has prognostic implications. Hyperkalemia results from the shift of potassium from inside to outside the cell, so insulin-dextrose infusion is the best treatment; calcium administration is not recommended as it may potentiate DADs.
Administration of digoxin-specific Fab antibody, if available, should always be attempted, especially in the presence of severe rhythm disturbances. Data from in vitro and animal models confirm that digoxin-specific Fab antibody cross-reacts with CAS.5–7 Clinical data, although not always demonstrating a clear benefit, supports its use and lack of harm. In a prospective study of CAS poisoning, Eddleston et al. found that its use decreased mortality by half when administered to patients exposed to yellow oleander (Thevetia peruviana).1 Dosage should not be based on digoxin concentration because of partial cross-reactivity, and large and repeated doses are recommended (190-380 mg, 30 min to 1 h intervals).
In our opinion, given the high mortality rate and the increasing recreational use of these substances, physicians working in the emergency department and intensive care should suspect CAS exposure when symptoms and electrocardiogram findings are reminiscent of digoxin toxicity.
To the best of our knowledge, this is the first fully electrocardiographically documented case of toxicity following intake of ‘Jamaican Stone’.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.