Increased thickness of left ventricular walls is the predominant characteristic and one of the diagnostic criteria of hypertrophic cardiomyopathy (HCM). This case illustrates an uncommon but important finding of isolated hypertrophy of the papillary muscles (PMs), observed in a young woman in whom an abnormal electrocardiogram was initially detected. During the investigation isolated PM hypertrophy was identified. The structural characteristics of the PMs have received scant attention in this setting and there is little information in the literature on this entity, whose real prevalence and clinical significance remain to be determined. The available information relates solitary PM hypertrophy with an early form or a different pattern of HCM. In this case PM hypertrophy was only detected due to the finding of an abnormal electrocardiogram, which prompted further diagnostic tests and a search for possible etiologies.
O aumento da espessura das paredes do ventrículo esquerdo é a característica predominante e um dos critérios de diagnóstico da miocardiopatia hipertrófica (MH). Este caso relata um achado incomum, mas importante, de hipertrofia isolada dos músculos papilares (MP), observada numa mulher jovem a quem foi inicialmente detetado um eletrocardiograma anormal. Durante a investigação realizada foi identificada uma hipertrofia isolada dos MP. As características estruturais dos MP têm recebido pouca atenção neste contexto. Existe insuficiente informação na literatura sobre esta entidade, cuja prevalência e relevância clínica se encontram por determinar, mas a informação disponível relaciona a hipertrofia isolada dos MP com uma forma precoce ou um padrão diferente de MH. Neste caso, a hipertrofia dos MP só terá sido detetada, provavelmente, devido ao achado de um eletrocardiograma anormal, o que orientou para a realização de exames complementares de diagnóstico adicionais e para a procura de possíveis etiologias.
The clinical diagnosis of hypertrophic cardiomyopathy (HCM) is conventionally made with cardiac imaging, at present most frequently two-dimensional echocardiography, but the use of cardiac magnetic resonance (CMR) imaging is increasing. According to the 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy, the morphologic diagnosis of HCM is based on the presence of a hypertrophied and nondilated left ventricle in the absence of another cardiac or systemic disease capable of producing the magnitude of that hypertrophy (usually ≥15 mm in adults or the equivalent relative to body surface area in children). There are also patients who are genotypically positive but phenotypically negative.1 The cardiac phenotype of HCM shows great diversity in the degree and pattern of hypertrophy (asymmetric, concentric, or apical), age of onset and clinical course.2 Although the papillary muscles (PMs) are an anatomic part of the left ventricular (LV) chamber, the significance and diverse morphology of these structures in HCM has not been systematically characterized.3 Papillary muscle (PM) hypertrophy is a rare echocardiographic finding, with very few cases reported in the literature.4 In an analysis of 6731 echocardiographic studies, Kobashi and colleagues5 found 29 patients with solitary PM hypertrophy, defined as either the vertical or horizontal diameter of at least one of the two PMs more than 11 mm (measured in end-diastole). Some of these patients presented electrocardiographic findings, such as high left precordial voltage and inverted T waves, very similar to those of apical hypertrophy. There are reports in the literature suggesting that PM hypertrophy, especially of the posteromedial muscle, could play an important role in the development of negative precordial T waves.5,6 There are also a few cases described of a diagnosis of HCM without hypertrophy at necropsy after sudden death, but the PMs were not described in the reports of these patients, so there is no information about the possible presence of hypertrophy of these structures.7 Therefore, solitary PM hypertrophy can have clinically important implications for the screening of HCM as a newly identified subtype of or an early form of HCM.5,8,9
CMR provides complete tomographic imaging of the heart with high spatial resolution images, and is thus an excellent imaging method to assess the PMs with precision.3 It is also a useful tool for further investigation as an established exam for assessment of different types of cardiomyopathies, since there are some typical findings in this exam that may suggest a particular pathology or etiology.10
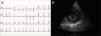
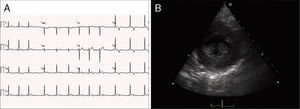
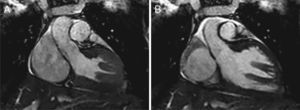
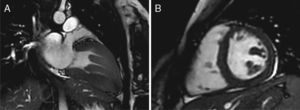
Case reportA 19-year-old woman presented palpitations and chest discomfort, unrelated to exertion and with no pleuritic characteristics, without reports of syncope, during the previous year. She had no relevant medical history and no known relevant family history of cardiac, renal, neurologic or genetic diseases; she was not under any medication and did not practice any sports. Her family physician performed a physical exam that was unremarkable (including blood pressure), except for an arrhythmia on cardiac auscultation. He requested an electrocardiogram (Figure 1A) which revealed sinus arrhythmia, biphasic T waves in leads DII, aVF and V3, and T-wave inversion in leads DIII and V4–V6. On the basis of this abnormal ECG clinical observation by a cardiologist was requested, in which the patient underwent more diagnostic exams. The echocardiogram revealed no significant abnormalities, except the presence of hypertrophic PMs (Figure 1B); LV mass was normal and maximum ventricular wall thickness was 11 mm at the interventricular septum in parasternal long-axis view. There were no significant valvular abnormalities. Despite the presence of prominent PMs, no significant intraventricular gradient or LV outflow tract obstruction was found on Doppler evaluation at rest. For further clarification and to assist with differential diagnosis, CMR imaging was performed. This exam showed a normal right ventricle and normal valve structures. The left ventricle had normal systolic function (60% ejection fraction), with a mass of 59 grams (35.5 g/m2), end-systolic volume of 42 ml and end-diastolic volume of 104 ml. Maximum ventricular wall thickness was 11.2 mm at the interventricular septum measured in end-diastole, 4-chamber view. Both PMs were also seen to be abnormally hypertrophic (Figures 2 and 3), occupying a large part of the LV cavity during systole (Figures 2A and 3A). The anterolateral PM had a maximum diameter of 12.5 mm and the posteromedial PM measured 15 mm on the horizontal axis (end-diastole, short-axis view; Figure 3B). Delayed hyperenhancement was not observed.
(A) 12-lead electrocardiogram showing the repolarization abnormalities described in the text. The voltage sum of the S wave in V1 and the R wave in V5 is 36 mm, but this patient was 19 years old, so the voltage criterion for left ventricular hypertrophy of ≥35 mm is not applicable; (B) echocardiographic image at the level of the papillary muscles (short-axis view at end-systole).
Laboratory tests were performed, but the results were unremarkable (including complete blood count, liver and kidney function tests, serum levels of muscle enzymes and urine analysis). In exercise stress testing the patient presented normal blood pressure and heart rate response; no arrhythmic events or other abnormalities were recorded. Twenty-four-hour Holter monitoring only showed the known sinus arrhythmia. At present the patient is not under any medication. She will be followed as an outpatient for further investigation, which will include stress echocardiography and genetic testing, since one of the most likely hypotheses is that this case may be an atypical presentation of HCM, with PM hypertrophy, considering the information available in the literature and the results of diagnostic tests. It will be interesting to assess the evolution of this patient over the coming years (especially regarding cardiac imaging). The patient was informed of her condition.
DiscussionThe clinical significance of abnormalities and hypertrophy of the PMs is a matter of debate and this morphological finding requires further investigation, as there are few articles published and little information on this entity.8
The published literature suggests that morphological abnormalities in PMs, such as anomalous insertion, are found in the context of LV wall hypertrophy, and that they are not uncommon in HCM, especially in apical hypertrophy. But they have also been reported as the only morphological abnormality in a subgroup of patients with HCM. It has also been suggested that isolated PM hypertrophy is a possible variant of HCM.5 These changes may evolve, along with progressive hypertrophy, over time.8 The recent American Heart Association classification of cardiomyopathies characterized HCM as a disease morphologically limited to the LV wall.1 However, other studies, including a relatively recent report showing extension of the hypertrophic process into the right ventricular wall in some patients,11 as well as the data presented here and in previous articles on PMs, show that the definition of HCM and its morphologic processes need to be broadened.3,9 More studies and investigation are needed to better understand this entity and to establish consensual criteria for measurement of PMs. On the basis of available information, the authors consider that this case may be a variant of HCM or an initial stage of the disease. However, taking into account the patient's medical history (including absence of family history of cardiac, renal, neurologic or genetic disease) and physical examination, other hypotheses were considered. Because of the repolarization alterations,12 pericarditis could be one differential diagnosis, but it did not fit in this context; there was no history of typical chest pain or an infectious disease, and the patient had two similar ECGs, with a five-week interval. ‘Athlete's heart’ did not seem a likely diagnosis, as the patient did not practice any kind of sports. Hypertension was also excluded. The patient denied consumption of drugs and was not under any kind of medication. Laboratory exams showed no relevant abnormalities. The relevant echocardiographic alterations were the PMs, which were visibly hypertrophic; the maximum wall thickness was 11 mm at the septum, and valvular disease was definitively excluded. After this abnormality was identified, the differential diagnosis became even more problematic. Fabry disease was one possibility,13 but although a genetic test has not been performed, neither the patient nor her relatives had no physical signs or symptoms suggestive of Fabry disease, and her blood and urine tests were normal. CMR also enabled further investigation, as it is an established exam for the etiologic assessment of cardiomyopathies.10,14 For example, some patterns of delayed enhancement may suggest a particular type of cardiomyopathy or etiology.10 But in this case delayed enhancement was not observed.
Taking into account the patient's clinical history and physical exam and the results of the diagnostic tests, Fabry disease, mitochondrial and infiltrative disorders such as amyloidosis, storage diseases (Danon disease) and other genetic syndromes (Noonan syndrome) are less probable in this setting.10,12–14 The relevant abnormal findings in this patient are repolarization alterations and the hypertrophied PMs (with normal ventricular mass), in which hypertrophy has been associated, in some studies and case reports, with abnormal T-wave inversion and with a possible variant of HCM or an initial stage of this disease.4–6,8,9
Clinically, HCM is usually recognized by maximum LV wall thickness ≥15 mm, with thickness of 13–14 mm considered borderline, particularly in the presence of other compelling information (for example family history of HCM).1 Genetic testing for HCM is available as a clinical test, but it has limitations, since about 50% of patients (depending on the series) have an identifiable mutation, and a substantial proportion have variants in which the pathogenicity of the mutation is uncertain. A genetic diagnosis is obtainable in only 30% when sporadic disease is considered.15,16
Despite this, the next step will be to perform a genetic test, since this case may be an atypical presentation or initial stage of HCM.1 Stress echocardiography will also be important, to determine the presence of ventricular gradients. According to the results of genetic tests and the patient's clinical course, assessment and screening of her first-degree relatives may be considered.
ConclusionConsidering the available information on PM hypertrophy, the case presented may represent a gap in our knowledge of HCM. More investigation is needed, which may affect the definition of HCM and thus possibly affect the diagnosis, management and prognosis of this disease. With this case, particularly with its images, the authors wish to demonstrate that HCM is a complex disease that goes beyond the left ventricle walls, and that such cases should not be missed, especially given the availability of imaging methods such as echocardiography and CMR. Echocardiography has been available for many years and is performed every day all over the world, but in medicine, we sometimes only find if we search.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Center where the work was performed: Povisa Hospital, Vigo, Spain.