Amiodarone is the most potent antiarrhythmic drug available and is commonly prescribed to treat and prevent not only life-threatening ventricular arrhythmias but also atrial fibrillation (AF). The latest European Society of Cardiology AF guidelines state that amiodarone is recommended for long-term rhythm control in all AF patients but that other antiarrhythmic drugs should be considered first whenever possible, due to its extracardiac toxicity. In patients without significant or with only minimal structural heart disease, amiodarone is not listed as a possibility in their therapeutic scheme. Still, amiodarone is widely and liberally used, and is the most prescribed antiarrhythmic drug for patients with AF despite its high toxicity profile. Non-cardiovascular death was more frequent with amiodarone treatment than with a rate control strategy in AFFIRM, while meta-analyses suggest an association between amiodarone use in patients without structural heart disease and increased non-cardiovascular mortality. Severe or even fatal outcomes due to amiodarone may occur years after treatment initiation and are often not acknowledged by the prescribing physician, who may no longer be following the patient. The lack of widely accepted diagnostic criteria and symptom definitions may lead to underestimation of the incidence of severe side effects and of its toxicity. Unlike the underestimated risk of toxicity with amiodarone, severe complications associated with catheter ablation are usually directly ascribed to the treatment even by non-medical personnel, possibly resulting in overestimation of risks. This brief review will address the issue of amiodarone overuse and the frequent underestimation of its toxicity, while suggesting scenarios in which its use is entirely reasonable, and compare it with catheter ablation.
A amiodarona é o fármaco antiarrítmico mais potente de que dispomos e é frequentemente prescrita para tratar e prevenir não apenas arritmias ventriculares malignas, mas também fibrilhação auricular (FA). Nas mais recentes diretrizes da Sociedade Europeia de Cardiologia, a «Amiodarona é recomendada para o controlo do ritmo a longo prazo em todos os doentes com FA». No entanto, os autores afirmam que «outros fármacos antiarrítmicos devem ser considerados primeiro sempre que possível» atendendo à toxicidade extracardíaca da amiodarona. Em doentes sem cardiopatia estrutural significativa, a amiodarona não está sequer listada como uma possibilidade no seu diagrama terapêutico. Ainda assim, a amiodarona é amplamente prescrita e liberalmente utilizada, representando o fármaco antiarrítmico mais prescrito para doentes com FA, apesar do seu elevado perfil de toxicidade. A morte não cardiovascular foi mais frequente com a amiodarona do que com uma estratégia de controlo de frequência no estudo AFFIRM, enquanto dados meta-analíticos sugerem uma associação entre o uso de amiodarona em doentes sem doença cardíaca estrutural e o aumento da mortalidade não cardiovascular. Eventos adversos graves e mesmo fatais devidos à amiodarona podem ocorrer anos após o início do tratamento e, muitas vezes, não são reconhecidos pelo médico que prescreveu o fármaco que pode já não estar a seguir o doente. A falta de critérios de diagnóstico amplamente aceites pode levar a uma subestimativa da incidência de efeitos secundários graves e a uma perceção subestimada da sua toxicidade. Contrariamente ao risco subestimado de toxicidade da amiodarona, as complicações graves associadas à ablação por cateter são facilmente atribuíveis ao tratamento, mesmo por pessoal não médico, resultando numa perceção possivelmente sobrestimada dos seus riscos. Esta breve revisão abordará o problema do uso excessivo de amiodarona e a perceção por norma subestimada da sua toxicidade e sugerirá cenários em que a sua utilização é inteiramente razoável.
An obstetrician woke up on his 69th birthday with fever, general malaise and a sore throat. Two days later, as he developed shortness of breath, he was taken to hospital where an initial diagnosis of diffuse bilateral pneumonia was made. As no response to antibiotics was seen within 24-48 hours, amiodarone pulmonary toxicity was suspected and a subsequent lung biopsy confirmed the diagnosis. The patient had been on amiodarone 200 mg once a day for 10 months, following ablation for atrial fibrillation. He had no previous lung disease. Steroids did not improve the patient's condition and sadly he died after seven weeks in the intensive care unit. He had complained of a dry cough for 2-3 months, with no other symptoms. A thoracic computed tomography scan had been normal eight weeks prior to admission to the intensive care unit. Just two days prior to hospitalization he had enjoyed a wine-tasting gondola cruise with his family with no breathlessness whatsoever. His death certificate read “Accidental death due to amiodarone-induced pulmonary toxicity”.1
IntroductionAmiodarone is the most potent antiarrhythmic drug available. It is commonly used for the treatment and prevention of life-threatening ventricular arrhythmias and also atrial fibrillation (AF).2 It is the most effective antiarrhythmic drug for the prevention of AF episodes.3 Its effects result from not only its ability to block sodium, potassium and calcium channels but also from noncompetitive beta-blockade. The latest European Society of Cardiology guidelines (ESC) on the management of AF4 recommend amiodarone as the first and only antiarrhythmic drug choice for patients with heart failure (HF) with reduced left ventricular (LV) ejection fraction, while in HF patients with preserved LV function or those with significant coronary artery or valvular disease both amiodarone and dronedarone receive a class IA indication. However, although the ESC guidelines state that amiodarone is recommended for long-term rhythm control in all AF patients, they also state that, owing to its extracardiac toxicity, other antiarrhythmic drugs should be considered first whenever possible. In patients without significant or with only minimal structural heart disease, who represent a large proportion of those admitted with AF and potentially eligible for catheter ablation, amiodarone is not considered in the new ESC guidelines as a treatment option, as seen in Figure 19 of the guideline document.4 Alternatives such as class Ic antiarrhythmic drugs like flecainide or propafenone, or other class III drugs such as dronedarone, should be considered prior to amiodarone, although physicians need to be aware that these drugs are not as effective as amiodarone and have their own disadvantages. Still, amiodarone is widely and liberally used, and is the most prescribed antiarrhythmic drug for patients with AF, with more than one fifth of paroxysmal AF patients and almost one third of those with persistent AF put on this drug5,6 despite its high toxicity and the availability of more effective interventional treatment.
Amiodarone use requires clear knowledge of its pharmacokinetics and potential for serious adverse events and drug interactions, as well as an understanding of available alternatives. This brief review will address the issue of amiodarone overuse and the frequent underestimation of its toxicity, while suggesting scenarios where its use is entirely reasonable, and compare it with catheter ablation.
Brief overview of amiodarone pharmacokineticsAmiodarone is highly lipophilic with a large volume of distribution, reflecting multi-compartmental distribution kinetics resulting in delayed onset of action and a long elimination half-life that can extend up to six months.7 It is metabolized to desethylamiodarone in the liver and has no significant renal metabolism. This results in delayed clinical effects when the drug is given in tablet form and diminishes its practical efficacy in pill-in-the-pocket scenarios, for which there are faster alternatives such as class Ic antiarrhythmic drugs.8,9 These considerations further extend the time during which the patient is exposed to potential serious side effects after treatment cessation. Although discontinuation of chronic amiodarone produces a prompt decrease in its serum concentrations, a low-concentration plateau persists for weeks to months due to the drug's slow release from tissue deposits.
Amiodarone-induced toxicity and adverse eventsAmiodarone has been associated with toxicity affecting the thyroid, lungs, liver, eyes, skin and the central and peripheral nervous systems, with a rate of adverse events as high as 15% in the first year and up to 50% with long-term use.10,11 Most adverse events are related to cumulative amiodarone exposure and are relatively manageable, subsiding with drug discontinuation, but some can persist even months after treatment is stopped or lead to life-threatening events. The end-organ accumulation of amiodarone is directly related to fat distribution, with the lungs and liver therefore being among the organs with the highest degree of drug accumulation.
Pulmonary toxicity, including hypersensitivity pneumonitis, alveolar/interstitial pneumonitis or fibrosis, pleuritis and bronchiolitis obliterans organizing pneumonia, can occur in up to 10-15% of cases and lead to a fatal outcome in cases when the diagnosis is missed in its early stages, a concerning possibility as the diagnosis is challenging. Although the highest incidence, of up to 15%, was reported in patients taking higher doses, pulmonary toxicity may occur at the standard 200 mg/day dosage12 or even after relatively short periods of treatment with lower doses.13,14 Amiodarone-induced pulmonary toxicity can occur with exposure at all doses, more commonly with increasing age and in those with chronic obstructive pulmonary disease and renal disease, and can result in astonishing crude mortality rates exceeding 75%.15 Amiodarone-induced hepatitis, seen in less than 3% of cases,16 can usually be easily recognized and acted upon in the presence of abnormal liver function tests, but the rarer diagnosis of amiodarone-induced cirrhosis carries a more ominous prognosis, with mortality as high as 60% at five months once the diagnosis is established.17 Although hypothyroidism is usually more common in iodine-sufficient populations, amiodarone-induced thyrotoxicosis may occur in up to 3% of cases and arises either from iodine-induced excessive thyroid hormone synthesis or destructive thyroiditis with release of preformed hormones. While hypothyroidism is usually an early event, cases of thyrotoxicosis may occur at any time during therapy, or often following discontinuation, and its development is unpredictable and can be of sudden onset.18 The latter can occasionally lead to a fatal outcome, especially in patients with LV impairment.19 Misdiagnosis or late diagnosis is possible as amiodarone itself along with the concomitant use of beta-blockers can inhibit adrenergically mediated signs of hyperthyroidism. Other potential side effects include gastrointestinal symptoms, skin discoloration and photosensitivity, optic neuritis, photophobia and corneal microdeposits, ataxia, tremor, peripheral polyneuropathy and impaired memory, bradycardia and prolonged QT interval.
Association between amiodarone and non-cardiovascular mortalityOverall death, intensive care unit stay, and non-cardiovascular death were more frequent with amiodarone treatment than with a rate control strategy in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial.20 A network meta-analysis of 39 randomized trials suggested a trend for increased mortality with amiodarone compared with placebo,21 while an association between amiodarone use in patients without structural heart disease and increased non-cardiovascular mortality has also been shown.22 However, the fatality rate associated with the most severe side effects of amiodarone remains unknown. Severe or even fatal outcomes due to amiodarone may occur years after treatment initiation and are often not acknowledged by the prescribing physician, who may no longer be following the patient. The lack of widely accepted diagnostic criteria and symptom definitions may lead to underestimation of the incidence of severe side effects and of its toxicity. Mortality in amiodarone pneumonitis is substantial and may range between 21% and 33% of patients who are admitted to the hospital,23–25 with many others suffering persistent sequelae. The diagnosis requires exclusion of other possible causes, often a lung biopsy and the observation of improvement following cessation of amiodarone, but a definite diagnosis is sometimes not reached and the drug may not be implicated, with certification ascribing death to pneumonia. Postoperative adult respiratory distress syndrome may occur in patients following cardiothoracic surgery who receive short courses of amiodarone, but again causality is often unclear.26,27 Amiodarone-induced cirrhosis, as well as thyrotoxicosis, may lead to fatal outcomes, particularly when these events are misdiagnosed.
A potential association between amiodarone and cancer has been raised by the results of a meta-analysis of four trials28 and a subsequent large population-based cohort study.29 In the former, cancer deaths were more common in amiodarone patients (0.7% vs. 0.02%) but still very infrequent,28 while in the latter the increased risk of cancer was restricted to male individuals and/or those taking higher daily doses.29 However, some potential risk factors for malignancy, such as obesity, smoking and family history, were not available in the study by Su et al.29 The evidence in the literature regarding amiodarone as a carcinogen is not only weak but also conflicting, with a more recent observational study finding no evidence of a dose-response relationship between cumulative amiodarone dose and incident cancer risk.30 It should be pointed out that the higher non-cardiovascular mortality in patients randomized to antiarrhythmic drug therapy in the AFFIRM trial was mostly due to pulmonary and cancer-related deaths,31 but a chance relationship between the rhythm-control strategy and cancer deaths could not be excluded.
Death indirectly caused by amiodarone may also result from interactions with other drugs, most commonly with warfarin. Amiodarone use is associated with significantly increased stroke and systemic embolism risk and a shorter time in therapeutic range when used with warfarin,32 and so alternative anticoagulants should be considered. It is possible that less effective anticoagulation with concomitant amiodarone treatment could contribute to the observed association with stroke or systemic embolism in warfarin-treated patients,32 but this remains speculative and may seem paradoxical considering that amiodarone potentiates the effect of warfarin, prolonging the international normalized ratio and thereby increasing the risk of bleeding.
In summary, although amiodarone does not seem to increase cardiac mortality, and thus represents the best antiarrhythmic drug choice in the HF setting, the existing evidence suggests that it may increase non-cardiovascular mortality.
Catheter ablation: an alternative or preferable to amiodarone?Catheter ablation for AF is becoming more widespread for numerous reasons and is now supported by evidence for benefit from both observational studies and randomized trials. Ablation is more effective than antiarrhythmic therapy in reducing the burden of AF and AF-related hospitalization, improving quality of life and delaying the progression of paroxysmal AF to more persistent forms.33–35 There is evidence for a survival benefit in HF patients with LV systolic dysfunction.33,36–38 Very recent research suggested that an early rhythm control strategy with antiarrhythmic drugs and/or catheter ablation in AF patients with acute strokes reduces the risk of recurrent stroke.39 The benefit was already noticeable within 3 months of the index stroke but became even more pronounced after 12 months.39 Still, no study has yet shown that AF ablation specifically reduces the risk of cardioembolic stroke in patients who never had a stroke. Although it may seem logical that AF ablation should reduce the risk of AF-related stroke, this effect should not be necessarily expected in adequately anticoagulated patients, for reasons that have been extensively discussed.40 Technologies used for AF ablation have improved progressively over the last two decades, and along with increasing operator experience have led to reductions in AF recurrence, complications, fluoroscopy use and radiation burden despite a shift toward more complex procedures.41–43 Moreover, the advent of single-shot techniques and improved analytics has improved the reproducibility of pulmonary vein isolation.44,45
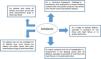
Accordingly, patients who are put on amiodarone as a long-term strategy should be informed of the potential for toxicity associated with this drug, its inferior efficacy compared with ablation, and the possibility that long-term amiodarone treatment may result in reduced efficacy of catheter ablation if and when patients are finally referred for this treatment, given the likely progression of the condition. Those with HF and LV impairment should also be informed of the potential for prognostic benefit with ablation compared with amiodarone.33,36–38 In contrast to the underestimation of the toxicity risk of amiodarone, severe complications associated with catheter ablation are usually directly ascribed to the treatment even by non-medical personnel, possibly resulting in overestimation of risks. Patients can die as a result of AF ablation, but reassuringly the procedure is now basically safe when performed in experienced centers.33 Whether the risk of procedure-related mortality exceeds that of amiodarone-induced toxicity is undetermined, as this has never been assessed. While the former is concentrated around the periprocedural period (with death due to atrioesophageal fistula occurring up to six weeks after ablation), amiodarone toxicity can lead to death years after initiation of treatment and even months after its discontinuation. Figure 1 illustrates the pros and cons of amiodarone compared to catheter ablation in terms of short- and medium- to long-term effects, respectively.
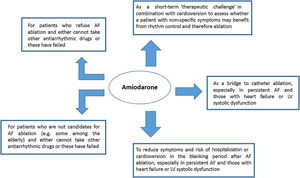
Amiodarone: the most effective antiarrhythmic drug is still a valid choice for someThe use of amiodarone for the treatment and prevention of AF is reasonable in some circumstances according to our experience (Figure 2): first, to provide symptom relief and prolong time in sinus rhythm as a bridge to catheter ablation, particularly when the waiting list for this intervention is longer than desired; second, as a short-term ‘therapeutic challenge’ in combination with electrical cardioversion to assess whether a patient with non-specific symptoms may benefit from rhythm control and therefore ablation; third, to provide symptom relief and reduce hospitalization and cardioversion rate during the blanking period after AF ablation,46 when patients may experience symptoms due to inflammation-induced atrial arrhythmias; fourth, to facilitate catheter ablation by reducing the duration of the procedure and ablation time, although this remains controversial as no long-term benefit has been demonstrated47,48; and finally, to reduce AF burden in patients who are not candidates for AF ablation or have a preference for antiarrhythmic drug therapy and in whom the benefits of a rhythm control strategy are thought to outweigh the risk of toxicity associated with medium- to long-term amiodarone treatment.
Amiodarone is the best antiarrhythmic drug choice for use in patients with structural heart disease or congestive HF given its good safety profile with regard to pro-arrhythmia49 and the potential risks associated with most other drugs in this context. It neither increases cardiac mortality in HF patients49 nor depresses ventricular function and thus can be administered empirically without the need for hospitalization. This is reflected in the latest ESC AF guidelines, in which amiodarone is the only recommended antiarrhythmic drug choice for patients with HF, while it is not listed as a recommendation in patients with a structurally normal heart.4 As a broad-spectrum multi-channel blocker, it has shown effectiveness against virtually the entire spectrum of cardiac tachyarrhythmias. Even so, care should be taken to use the lowest possible dose, as AF may be reasonably well controlled in many patients using a forced down-titration monitoring protocol with few side effects over a long follow-up.50 Low-dose amiodarone also displays a low incidence of significant side effects requiring medication discontinuation,51 although the therapeutic efficacy of routine low-dose amiodarone is unclear and toxicity may still occur. Also, periodic assessment is warranted with thyroid and liver function tests (every six months), chest X-ray (every 12 months), pulmonary function tests and ophthalmologic examination (if symptoms develop).52,53 There is some evidence that exposure to supplemental O2, especially at high concentrations, alone or when combined with mechanical ventilation, may potentiate progression of diffuse pneumonitis with acute respiratory failure, and this should be taken into account by anesthetists when assessing perioperative risk, particularly for patients scheduled for cardiothoracic surgery.26,27
Gaps in the literatureMost data on amiodarone-induced toxicity are from two decades ago. The current incidence of severe amiodarone toxicity and related mortality remain unclear. It is plausible that severe side effects of amiodarone have become increasingly recognized in recent decades, enabling earlier diagnosis. Even so, deaths due to ‘pulmonary failure’ or ‘pneumonia’ in patients taking amiodarone should be more thoroughly scrutinized, as the drug itself may have caused some of them. Whether amiodarone-related fatalities exceed those directly caused by AF ablation has never been analyzed, but such an investigation would be worthwhile in order to place competing risks in context. Long-term registries of patients under amiodarone would also help to assess rates of cumulative side effects.
More studies on patients’ preferences concerning ablation versus drug therapy are also clearly needed. The limited literature on this suggests that few AF patients really do maintain quality of life over time despite active long-term antiarrhythmic drug treatment, while most still prefer what they perceive as sinus rhythm even at the cost of accepting an invasive treatment procedure, regardless of AF symptom severity and underlying comorbidities.54 This assessment was based on a 2007 questionnaire,54 and it is likely that an even higher percentage of patients would currently favor invasive treatment considering the improvements in procedural success and safety seen over the last two decades, in contrast with the stagnation seen in the field of antiarrhythmic drug treatment.
It is not uncommon for AF patients to be oblivious of the possibility of ablation even years after the diagnosis. However, many patients who are put on drug therapy would have preferred an ablation if they had been involved in informed decision-making. Although providing patients with complete information in a format which they can easily access may be challenging, empowering patients to be effective advocates for their health requires thorough understanding of available treatments.
ConclusionAmiodarone is the most effective antiarrhythmic drug choice to control AF when catheter ablation is deemed too risky or patients need short-term symptom control. Nevertheless, its efficacy is inferior to that of ablation and its potential for toxicity is higher than is generally perceived by physicians. Patients should be clearly informed of the risks associated with amiodarone, as well as its efficacy in rhythm control, in comparison with those of catheter ablation, so that a truly informed decision can be made.
Conflicts of interestS. Barra has received training grants from Biosense and Biotronik. S. Boveda has received consulting fees from Medtronic, Boston Scientific and Microport.