The prognosis of chronic heart failure with reduced ejection fraction (HFrEF) has been studied extensively, but factors predicting cardiac decompensation are poorly defined. Right ventricular stroke work index (RVSWI), an invasive measure of right ventricular (RV) systolic function, is a well-known prognostic marker of RV failure after left ventricular assist device insertion and after lung transplantation. Thus, the aim of this study was to assess whether there is a relationship between RVSWI, HFrEF hospital readmission due to cardiac decompensation, and prognosis.
MethodsWe prospectively enrolled 132 consecutive patients with HFrEF. Right heart catheterization was performed and RVSWI values were calculated in all patients. The relationship between RVSWI values and readmission and prognosis was analyzed.
ResultsDuring a median follow-up of 20±7 months, 33 patients were readmitted due to cardiac decompensation in the survivor group, and 18 patients died due to cardiac causes. There was no difference between patients who died and survived in terms of RVSWI values. Among patients with decompensation, mean RVSWI was significantly lower than in patients with stable HFrEF (6.0±2.2 g/m2/beat vs. 8.8±3.5 g/m2/beat, p<0.001). On correlation analysis, RVSWI was negatively correlated with NYHA functional class. RVSWI was also identified as an independent risk factor for cardiac decompensation in Cox regression survival analysis.
ConclusionsWe showed that RVSWI predicts cardiac decompensation and correlates with functional class in advanced stage HFrEF. Our data suggest the value of combining information on right heart hemodynamics with assessment of RV function when defining the risk of patients with advanced HFrEF.
O prognóstico de insuficiência cardíaca crónica com fração de ejeção reduzida (ICFER) tem sido amplamente estudado, mas os fatores prognósticos de descompensação cardíaca estão mal definidos. O índice de trabalho sistólico ventricular direito (ITSVD), um indicador invasivo da função sistólica do ventrículo direito (VD), é um marcador prognóstico bem conhecido de falência VD após inserção de dispositivos de assistência ventricular e após transplante pulmomar. Deste modo, o objetivo deste estudo consistiu em avaliar se existe relação entre o ITSVD e os reinternamentos por ICFER e entre este índice e o prognóstico.
MétodosEfetuámos o estudo prospetivo de 132 doentes consecutivos com ICFER. Foi realizado cateterismo direito, tendo o ITSVD sido calculado em todos os doentes. Foi avaliada a relação dos valores de ITSVD com o reinternamento e com o prognóstico da doença.
ResultadosDurante um período de seguimento médio de 20±7 meses, no grupo de sobreviventes, 33 doentes foram reinternados devido a descompensação cardíaca e 18 doentes morreram por causa cardíaca. Não se verifcaram diferenças quanto aos valores do ITSVD entre os doentes que morreram e os que sobreviveram. Nos doentes com descompensação cardíaca, o valor médio de ITSVD foi significativamente inferior ao dos doentes com ICFER clinicamente estáveis (6,0±2,2 g/m2/batimento versus 8,8±3,5 g/m2/batimento, p<0,001). O ITSVD correlacionou-se inversamente com a classe funcional de NYHA e revelou ser um fator de risco independente de descompensação cardíaca na análise da regressão de Cox.
ConclusõesO ITSVD prevê a descompensação cardíaca e correlaciona-se com a classe funcional NYHA em estádios avançados de ICFER. Os nossos dados sugerem a importância de combinar informação sobre a hemodinâmica do coração direito com a avaliação funcional do VD para tentar definir o risco dos doentes com ICFER avançada.
Heart failure with reduced ejection fraction (HFrEF) is a progressive condition, beginning with predisposing factors and leading to the development and worsening of clinical illness. Mortality is high and hospitalization rates for HFrEF have increased in recent years.1 Although many studies have demonstrated that left ventricular ejection fraction (LVEF) is associated with adverse prognosis, in recent reports there is controversy about the prognostic significance of LVEF, especially in the advanced stages of HFrEF. The prognostic utility of LVEF and its relation with symptomatic progress in fact decline once its value falls below 25%.2–4 Recently, there has been increasing evidence that right ventricular (RV) systolic function is a more powerful predictor of mortality in this subgroup of patients. A number of studies have provided evidence that RV ejection fraction, measured by thermodilution during right heart catheterization or indirectly by echocardiography, is an independent prognostic factor in severe HFrEF.5,6 The absence of a universally accepted standard clinical method for assessing RV systolic function stems from its dependence on afterload of this chamber. An ideal indicator of myocardial contractility should be independent of preload, afterload and heart rate. Right heart catheterization has long been a routine investigation in advanced HFrEF, and its measurements provide valuable information on prognosis, especially in the advanced stages of HFrEF; therapy guided by invasive hemodynamic monitoring and clinical assessment together improves clinical outcomes over therapy guided by expert clinical assessment alone.7,8 Right ventricular stroke work index (RVSWI), an invasive hemodynamic parameter, measures systolic function and its significant sensitivity to inotropy helps assess myocardial contractility. Also, indexing stroke work reduces the influence of loading conditions on measurement of systolic function.9
In the present study, we analyzed RVSWI as a marker for RV systolic function to predict acute decompensation of chronic heart failure (HF). In addition, we analyzed the relationship between RVSWI and progression of patients’ symptomatic status in terms of New York Heart Association (NYHA) functional class. Furthermore, this study provides additional information on planning strategies that aim to optimize the management of HFrEF in order to curtail costs by reducing hospitalizations.
MethodsPatient populationWe prospectively enrolled 132 consecutive patients (104 male, 28 female and age 24-81 years) with stable HFrEF, defined as LVEF≤35%. The study period was April 2011 to November 2012. Informed consent was obtained from all study patients and institutional ethics committee approval was granted. All patients were in severe HF as defined by the Framingham criteria, with an etiology of dilated cardiomyopathy or ischemic heart disease.
Dilated cardiomyopathy was defined as left ventricular dysfunction in the absence of significant coronary artery disease (≥70% luminal narrowing) on coronary angiography or absence of pathologic Q waves on electrocardiography, active myocarditis, or valvular heart disease. Ischemic heart disease was diagnosed on the basis of documented previous myocardial infarction or significant coronary artery disease on coronary angiography.
Patients with active myocarditis, amyloidosis, valvular heart disease, hypertrophic cardiomyopathy, active alcoholism, malignancy, active liver or kidney disease, myocardial infarction more than six months previously or unstable angina were excluded. Included patients were defined as having stable chronic HF.
Assessment and managementAll patients’ medical history, demographic characteristics, cardiovascular risk factors, comorbid illnesses, NYHA functional class, and echocardiographic exams were analyzed. Functional status at the time of referral was assessed according to the decision of the attending cardiologist.
EchocardiographyStandard grayscale, Doppler, and two-dimensional echocardiographic examinations were performed using a Vivid 3 ultrasound system equipped with a 3-MHz transducer and tissue Doppler imaging technology. All echocardiographic studies were performed with the subject in the left lateral decubitus position. At least two consecutive heartbeats were analyzed and mean values were considered. Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) diameter were calculated from M-mode recordings with the cursor placed at the tip of the mitral leaflets in parasternal long-axis view. Baseline RV end-diastolic and end-systolic diameter, right and left atrial diameter, and tricuspid annular plane systolic excursion (TAPSE) were measured in apical 4-chamber view, as previously described.10 LVEF was assessed using the biplane Simpson method in apical 4- and 2-chamber views. Pulmonary artery systolic pressure (PASP) was estimated by continuous-wave Doppler echocardiography of the tricuspid inflow.11
MedicationThe medical regimen was carefully assessed and optimized to standard HF treatment. The oral regimen was based primarily on angiotensin-converting enzyme inhibitors, aldosterone antagonists and beta-blockers. The loop diuretic regimen was adjusted to maintain the patient's daily weight at the level at which optimal hemodynamic data were achieved. Digoxin was prescribed for patients without contraindication. No investigational agent was used.
Right heart catheterizationAfter initial assessment of possible eligibility for transplantation, 132 patients underwent formal evaluation for transplantation or assist device, which included right heart catheterization to determine RVSWI. Investigations were performed via the right femoral vein.
RVSWI was calculated as follows:
RVSWI=(mean pulmonary artery pressure-mean right atrial pressure)×stroke volume index×0.0136
Stroke volume index=cardiac index/heart rate
Normal RVSWI values: 5-10 g/m2/beat.12–14
Follow-upThe patients were followed for changes in NYHA functional class and readmission for cardiac decompensation. Heart failure requiring hospitalization was identified as exacerbation of dyspnea, need for parenteral diuretic therapy, and symptoms associated with left or right HF. Survival was defined as freedom from cardiac-related death. Patients without any left or right HF symptoms were defined as having stable HFrEF.
Statistical analysisContinuous variables were expressed as mean ± standard deviation and categorical variables were expressed as frequencies. Continuous variables were compared between groups using analysis of variance (ANOVA), and categorical variables were compared using the chi-square test. Correlation analyses were performed by the Pearson rank test. One-way ANOVA was used to analyze more than two categorical variables. Survival was analyzed with Cox regression and Kaplan-Meier survival curves. A p-value <0.05 was considered statistically significant. IBM SPSS version 22.0 windows (IBM SPSS Inc., Chicago, IL) was used for analysis. Receiver operating characteristic curve analysis was used to determine the optimum cut-off values of RVSWI to predict the development of decompensation.
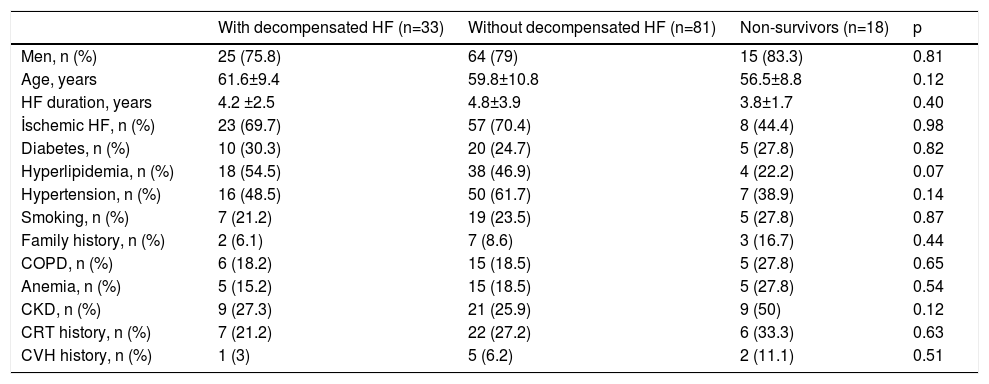
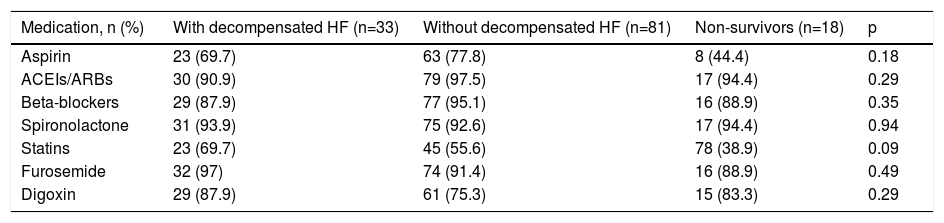
ResultsA total of 132 patients with advanced HF were included. There were no significant differences between the three groups (patients with decompensated HF, those without decompensated HF and those who died) in terms of gender, etiology of HF, cardiovascular risk factors or comorbidities (Table 1). All patients were on optimal medical therapy and there were no significant differences in medications between the three groups (Table 2).
Characteristics of the study groups.
| With decompensated HF (n=33) | Without decompensated HF (n=81) | Non-survivors (n=18) | p | |
|---|---|---|---|---|
| Men, n (%) | 25 (75.8) | 64 (79) | 15 (83.3) | 0.81 |
| Age, years | 61.6±9.4 | 59.8±10.8 | 56.5±8.8 | 0.12 |
| HF duration, years | 4.2 ±2.5 | 4.8±3.9 | 3.8±1.7 | 0.40 |
| İschemic HF, n (%) | 23 (69.7) | 57 (70.4) | 8 (44.4) | 0.98 |
| Diabetes, n (%) | 10 (30.3) | 20 (24.7) | 5 (27.8) | 0.82 |
| Hyperlipidemia, n (%) | 18 (54.5) | 38 (46.9) | 4 (22.2) | 0.07 |
| Hypertension, n (%) | 16 (48.5) | 50 (61.7) | 7 (38.9) | 0.14 |
| Smoking, n (%) | 7 (21.2) | 19 (23.5) | 5 (27.8) | 0.87 |
| Family history, n (%) | 2 (6.1) | 7 (8.6) | 3 (16.7) | 0.44 |
| COPD, n (%) | 6 (18.2) | 15 (18.5) | 5 (27.8) | 0.65 |
| Anemia, n (%) | 5 (15.2) | 15 (18.5) | 5 (27.8) | 0.54 |
| CKD, n (%) | 9 (27.3) | 21 (25.9) | 9 (50) | 0.12 |
| CRT history, n (%) | 7 (21.2) | 22 (27.2) | 6 (33.3) | 0.63 |
| CVH history, n (%) | 1 (3) | 5 (6.2) | 2 (11.1) | 0.51 |
CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronization therapy; CVH: cerebrovasculary disease; HF: heart failure.
Characteristics of medication in the study groups.
| Medication, n (%) | With decompensated HF (n=33) | Without decompensated HF (n=81) | Non-survivors (n=18) | p |
|---|---|---|---|---|
| Aspirin | 23 (69.7) | 63 (77.8) | 8 (44.4) | 0.18 |
| ACEIs/ARBs | 30 (90.9) | 79 (97.5) | 17 (94.4) | 0.29 |
| Beta-blockers | 29 (87.9) | 77 (95.1) | 16 (88.9) | 0.35 |
| Spironolactone | 31 (93.9) | 75 (92.6) | 17 (94.4) | 0.94 |
| Statins | 23 (69.7) | 45 (55.6) | 78 (38.9) | 0.09 |
| Furosemide | 32 (97) | 74 (91.4) | 16 (88.9) | 0.49 |
| Digoxin | 29 (87.9) | 61 (75.3) | 15 (83.3) | 0.29 |
ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
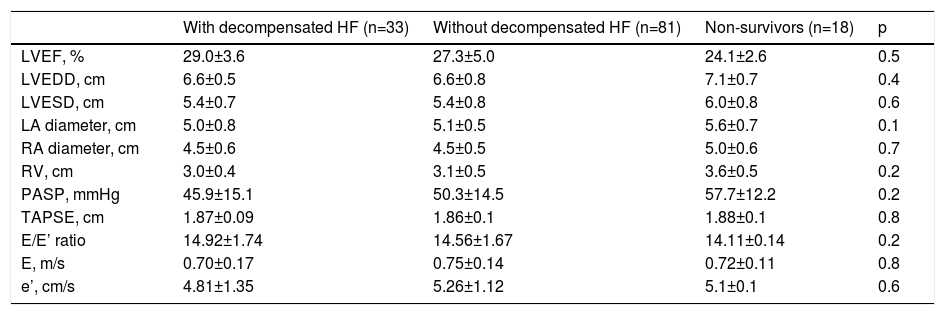
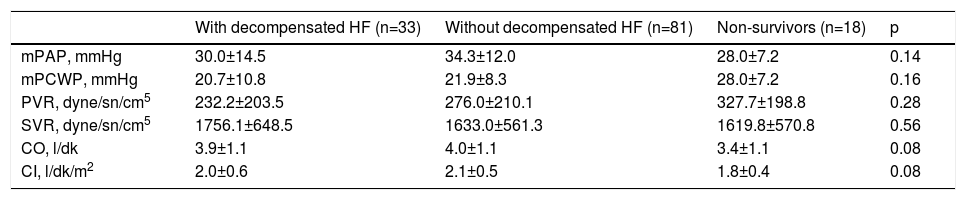
Echocardiographic findings and right heart catheterization parameters did not differ significantly between the three groups (Tables 3 and 4 and Figure 1).
Echocardiographic parameters in the study groups.
| With decompensated HF (n=33) | Without decompensated HF (n=81) | Non-survivors (n=18) | p | |
|---|---|---|---|---|
| LVEF, % | 29.0±3.6 | 27.3±5.0 | 24.1±2.6 | 0.5 |
| LVEDD, cm | 6.6±0.5 | 6.6±0.8 | 7.1±0.7 | 0.4 |
| LVESD, cm | 5.4±0.7 | 5.4±0.8 | 6.0±0.8 | 0.6 |
| LA diameter, cm | 5.0±0.8 | 5.1±0.5 | 5.6±0.7 | 0.1 |
| RA diameter, cm | 4.5±0.6 | 4.5±0.5 | 5.0±0.6 | 0.7 |
| RV, cm | 3.0±0.4 | 3.1±0.5 | 3.6±0.5 | 0.2 |
| PASP, mmHg | 45.9±15.1 | 50.3±14.5 | 57.7±12.2 | 0.2 |
| TAPSE, cm | 1.87±0.09 | 1.86±0.1 | 1.88±0.1 | 0.8 |
| E/E’ ratio | 14.92±1.74 | 14.56±1.67 | 14.11±0.14 | 0.2 |
| E, m/s | 0.70±0.17 | 0.75±0.14 | 0.72±0.11 | 0.8 |
| e’, cm/s | 4.81±1.35 | 5.26±1.12 | 5.1±0.1 | 0.6 |
LA: left atrial; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end systolic diameter; PASP: pulmonary artery systolic pressure; RA: right atrial; RV: right ventricular; TAPSE: tricuspid annular plane systolic excursion.
Invasive right heart catheterization parameters in the study groups.
| With decompensated HF (n=33) | Without decompensated HF (n=81) | Non-survivors (n=18) | p | |
|---|---|---|---|---|
| mPAP, mmHg | 30.0±14.5 | 34.3±12.0 | 28.0±7.2 | 0.14 |
| mPCWP, mmHg | 20.7±10.8 | 21.9±8.3 | 28.0±7.2 | 0.16 |
| PVR, dyne/sn/cm5 | 232.2±203.5 | 276.0±210.1 | 327.7±198.8 | 0.28 |
| SVR, dyne/sn/cm5 | 1756.1±648.5 | 1633.0±561.3 | 1619.8±570.8 | 0.56 |
| CO, l/dk | 3.9±1.1 | 4.0±1.1 | 3.4±1.1 | 0.08 |
| CI, l/dk/m2 | 2.0±0.6 | 2.1±0.5 | 1.8±0.4 | 0.08 |
CI: cardiac index; CO: cardiac output; mPAP: mean pulmonary artery pressure; mPCWP: mean pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; SVR: systemic vascular resistance.
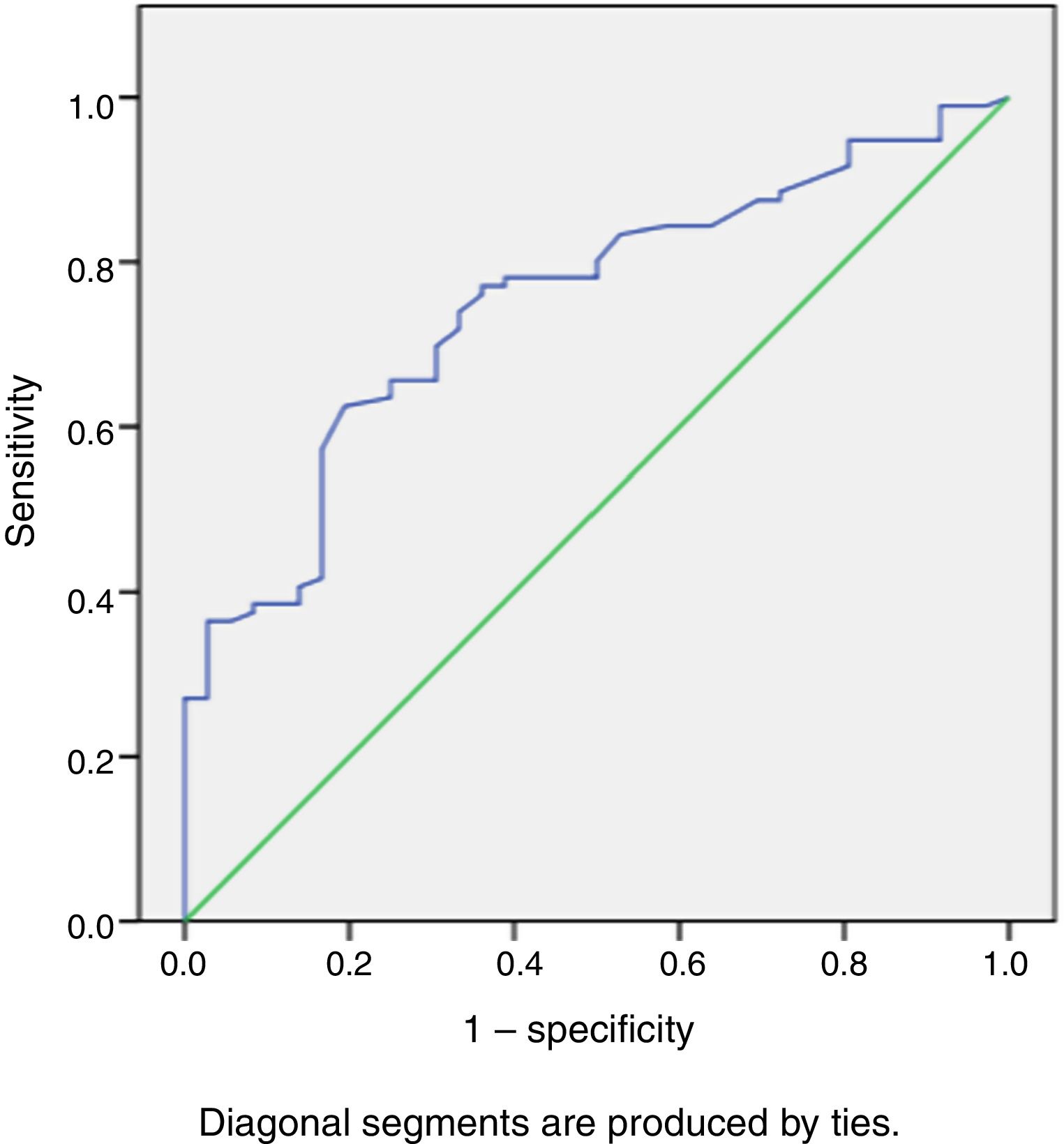
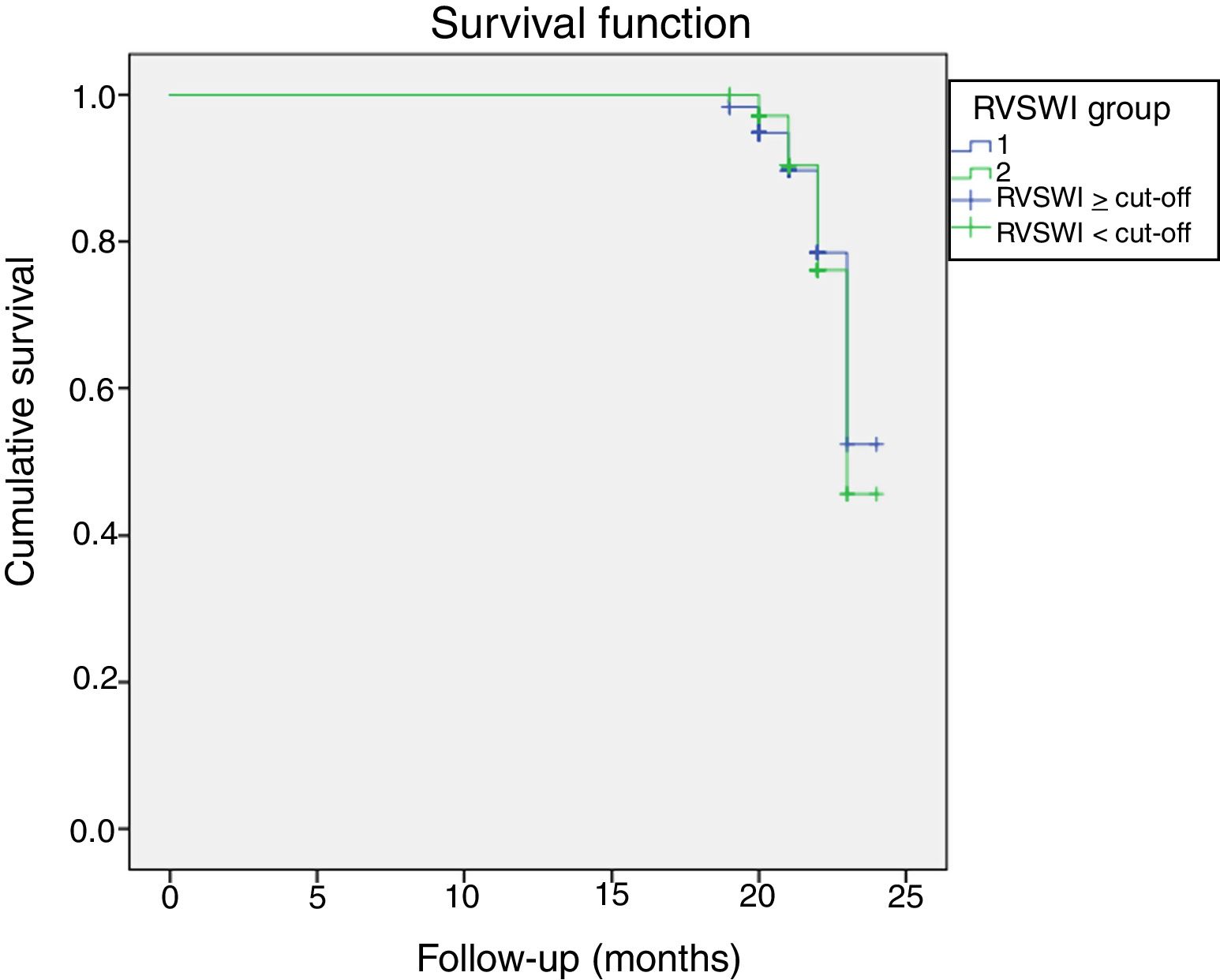
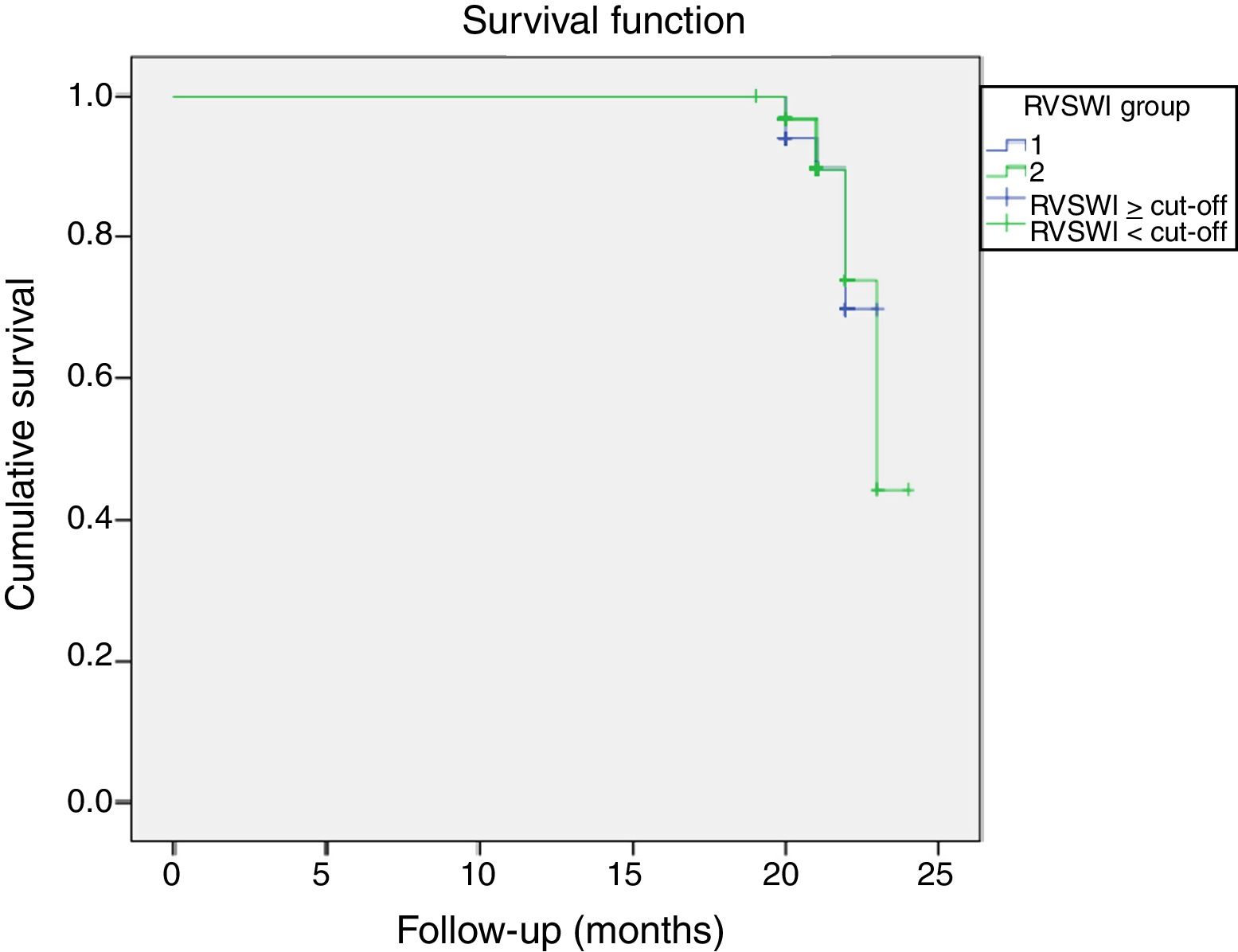
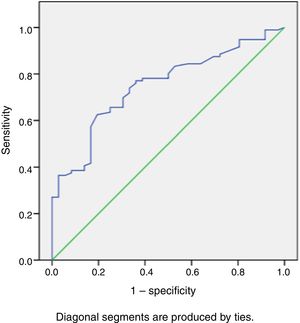
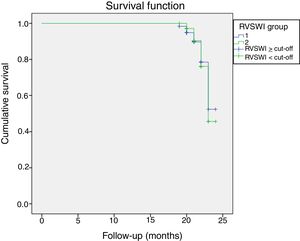
During a median follow-up of 20±7 months, 33 patients were readmitted due to cardiac compensation and 18 patients died due to cardiac causes. There was no significant difference in RVSWI values between patients who died and survivors (7.55±3.49 vs. 8.02±3.44, p: 0.59). Among patients with decompensation, mean RVSWI was significantly lower than in patients without decompensation (6.0±2.2 g/m2/beat vs. 8.8±3.5 g/m2/beat, p<0.001), and on correlation analysis, RVSWI was negatively correlated with NYHA functional class (r: -0.469, p<0.002). RVSWI was also identified as an independent risk factor for cardiac decompensation in Cox regression survival analysis (hazard ratio: 0.812, p=0.002, 95% confidence interval [CI]: 0.71-0.92). In receiver operating characteristic curve analysis, the area under the curve for predicting decompensation was 0.750 (p<0.001, 95% CI 0.66-0.84) and the cut-off for RVSWI was 7.25 (sensitivity 66%, specificity 75%) (Figure 2). Kaplan-Meier survival analysis showed no difference (with or without events during follow-up) between two groups divided by our RVSWI cut-off (Tables 5 and 6 and Figures 3 and 4). None of our patients underwent heart transplantation during follow-up.
In our study we showed that RVSWI was negatively correlated with NYHA functional class and was an independent risk factor for cardiac decompensation. The primary finding from this analysis is that higher RVSWI predicts lower rates of acute HF decompensation. Combining information on right heart hemodynamics with assessment of RV function helps to define the risk of patients with advanced HFrEF.
LVEF is a proven prognostic marker in HF. However, recent studies have revealed that the prognostic utility of LVEF and its relation with symptomatic progress decline once its value falls below 25%.2–4 LVEDD, RV function (RV fractional area change and TAPSE), restrictive mitral inflow filling pattern and pulmonary hypertension are also known risk factors in the prognosis of patients with systolic dysfunction and HF.15 The data suggest the value of combining information on right heart hemodynamics with assessment of RV function when defining the risk of patients with advanced HFrEF. A study on ambulatory HF patients showed that risk stratification based on exercise performance, left-sided congestion, RV dysfunction and liver congestion can help prediction of one-year prognosis.16 All these risk factors can be easily and non-invasively obtained and are useful for predicting prognosis in follow-up. However, our findings did not support this, showing that LVEF, TAPSE, E/E’ ratio and LVEDD values did not differ between decompensated and non-decompensated patients. Additionally, several known risk factors for HF readmission were not significantly different between the groups. In our daily cardiology practice, we find that patients with similar echocardiographic parameters and risk factors can have a very different course during follow-up. At this stage, invasive assessment is important for the follow-up and monitoring of these patients. In our study only RVSWI varied between the two groups and was associated with cardiac decompensation. RVSWI appears to be a more effective and objective method than TAPSE to demonstrate RV function.
Due to the geometric complexity of the right ventricle, assessment of RV function has historically been very difficult. Its shape cannot be explained by mathematical models and any method that relies on visual assessment, such as standard transthoracic echocardiography, is of limited use. Most studies in the literature are based on radionuclide ventriculography.17 RVSWI is an invasive hemodynamic parameter and data on its clinical use are lacking. Previous reports on RVSWI were mostly concerned with left ventricular assist device insertion,18 in which it was demonstrated to be a preoperative risk factor for RV failure after device insertion, or after lung transplantation, for which it was a predictor of mortality and length of hospital stay.19 In our study, we analyzed RVSWI as a marker for RV systolic function to predict acute decompensation of chronic HF. In addition, we assessed the relationship between RVSWI and patients’ symptomatic status in terms of NYHA functional class. RVSWI was also identified as an independent risk factor for cardiac decompensation. In the literature, RV dysfunction as defined by RVSWI is accepted to be below 5 g/m2/beat.19 Our study identifies a cut-off value for increased probability of HF decompensation.
The prognosis of chronic HF has been studied extensively, but factors predicting cardiac decompensation are poorly defined. Despite variations in study populations and methodologies of assessment, evidence of RV dysfunction predicts an inferior outcome. In previous studies, it was demonstrated that RV dysfunction is associated with poor survival, increased frequency of non-fatal cardiac events and worse functional class.6,17 Additionally, a recently published study demonstrated that compared with left ventricular stroke work, increasing RV stroke work during treatment of HF was associated with better outcomes.20 In our study, the relationship between RVSWI and HF decompensation rates provides direct evidence that RV dysfunction is linked to poor prognosis, providing valuable information for prediction of decompensation, especially in advanced stages of HF, in which the prognostic utility of LVEF is reduced. The finding that patients with better RV function have better symptomatic status is in accordance with previous studies.12,21 However, no study has assessed the direct relationship of RVSWI with acute HF decompensation. Furthermore, in previous studies RV functional assessments were either performed with non-invasive indices12 or, if invasive hemodynamic parameters were assessed, RVSWI was only one among several other prognostic indices.22 This is important because right heart catheterization has become a routine clinical exam in advanced stages of HF in order to plan treatment strategies such as transplantation or assist devices, and in contrast to most other load-dependent parameters, RV assessment by RVSWI provides direct information about prognosis and symptomatic progress independently.
In our study, RVSWI did not predict mortality. This was considered to be mainly due to the short follow-up duration. We also suggest that long-term follow-up studies may clarify the prognostic importance of RVSWI.
In previous studies, increased frequency of non-fatal hospitalization was found to influence subsequent HF mortality.23 Our study points to another aspect of this issue: that a higher RVSWI value is directly related to reduced frequency of HF hospital admissions, providing additional information for planning strategies that aim to curtail costs by reducing HF hospitalizations. The clinical implications of these findings are twofold. First, hospitalizations related to acute decompensated HF are associated with poor prognosis and reduced quality of life. Second, by predicting future hospitalizations due to decompensation, cost-saving disease management programs can be improved, since the total annual cost of care has been found to be high in cost analyses of acute decompensated HF.24
Ghio et al.14 analyzed the coupling between PASP and RV ejection fraction and its value in estimating the prognosis of patients with HF. Patients with high PASP and RV dysfunction had worse prognosis, and those with normal RV function had a similar prognosis to that of patients with normal PASP, irrespective of the etiology of HF. In contrast to this study, our data demonstrated no significant difference in the echocardiographic parameters of LVEF and PASP between the two patient groups, although there were significant differences in RVSWI values and decompensation outcomes.
Our study has some limitations. First, it was a single-center study. Second, the number of patients was relatively small, with an uneven, short follow-up, and there is a need for long-term follow-up for further elucidation. Finally, we did not assess pro-B-type natriuretic peptide levels.
ConclusionsRVSWI is a predictor of cardiac decompensation among patients with advanced HF. Our observations emphasize the need to combine invasive RV hemodynamic parameters with LVEF and functional class in the prognosis of HF when defining patients’ individual risk profiles. Risk models consisting of invasive, non-invasive and clinical parameters should be developed for prediction of prognosis, especially in the advanced stages of HF.
Conflicts of interestThe authors have no conflicts of interest to declare.