We present the first case of an iatrogenic aorta to right ventricular outflow tract fistula after Melody valve implantation. A 11-year-old girl, born with tetralogy of Fallot with absent pulmonary valve, underwent surgical repair at three years old with a 15-mm homograft. At five years old, calcification and stenosis of the homograft prompted successful balloon angioplasty and five years later she underwent Melody valve implantation. During follow-up, she began to suffer fatigue on moderate exertion. Echocardiography, cardiac catheterization and computed tomography were performed and showed a significant fistula between the right coronary ostium and the right ventricular outflow tract proximal to the implanted valve. The patient underwent surgical repair and in long-term follow-up there is no evidence of the fistula.
Iatrogenic fistula between the ascending aorta and the right ventricular outflow tract after percutaneous pulmonary valve implantation is an uncommon complication, and may grow over time. A high level of suspicion is required for this rare complication and a final aortography may be necessary for the diagnosis.
Com este caso clínico pretende-se reportar o primeiro caso de uma fístula iatrogénica da aorta para a câmara de saída do ventrículo direito após implantação de válvula Melody. Uma rapariga de 11 anos, com o diagnóstico de tetralogia de Fallot e agenesia da válvula pulmonar, submetida a correção cirúrgica total aos três anos com um homoenxerto de 15 mm, foi submetida, aos cinco anos, a angioplastia percutânea por estenose e calcificação do homoenxerto e aos 10 anos a implantação percutânea de válvula Melody. No seguimento em ambulatório, surgiram queixas de fadiga para esforços moderados. A doente realizou ecocardiograma, cateterismo cardíaco e tomografia computorizada que demonstraram existência de uma fístula entre o ostium coronário direito e a câmara de saída do ventrículo direito, com localização proximal à válvula implantada. A adolescente foi submetida a correção cirúrgica, verificando-se no seguimento a longo prazo ausência de fístula.
As fístulas iatrogénicas entre a aorta ascendente e a câmara de saída do ventrículo direito após implantação de válvula pulmonar percutânea podem crescer ao longo do tempo. Para o diagnóstico destas complicações raras é necessário um elevado nível de suspeição e eventualmente a realização de uma aortografia final.
Percutaneous pulmonary valve implantation (PPVI) is a nonsurgical and less invasive alternative for the treatment of right ventricular outflow tract (RVOT) dysfunction that is increasingly used.
Iatrogenic fistulas between the aorta and pulmonary artery (PA) are uncommon, and usually related to transcatheter intervention on the pulmonary circulation, such as balloon pulmonary angioplasty, PA stent implantation and in five cases following transcatheter pulmonary valve replacement.1
To our knowledge this is the first case report of an iatrogenic aorta to RVOT fistula after PPVI. The etiology and pathophysiology of this rare complication are clearly different from cases with an aortopulmonary fistula distal to the PPVI, as we present in this new report and literature review.
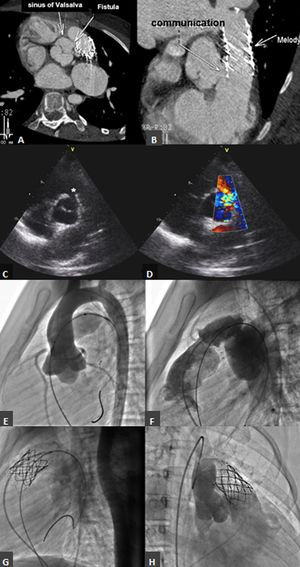
Case reportA 11-year-old girl, born with tetralogy of Fallot with absent pulmonary valve, underwent surgical repair at three years old with a 15-mm homograft. A small residual ventricular septal defect (VSD) was noted on follow-up. At five years old, calcification and stenosis of the homograft prompted successful balloon angioplasty, and at 10 years old she underwent PPVI under general anesthesia. Coronary contraindications were excluded with an aortic root angiography simultaneous with a fully inflated 20-mm Atlas Gold balloon (Bard Peripheral Vascular Inc, Tempe, AZ) (Figure 1E and F). Pre-stenting was performed with a 43-mm AndraStent XL (Andramed, Reutlingen, Germany) mounted on a 20-mm BiB catheter (Numed Inc, Hopkinton, NY), and a 22-mm Melody transcatheter pulmonary valve (Medtronic Inc, Mounds View, MN) was implanted with an Ensemble delivery system (Figure 1G). The final PA pressure was 28/11/16 mmHg, right ventricular (RV) pressure was 28/8 mmHg, aortic pressure was 77/45/60 mmHg and there was no RV-PA gradient. The exit angiogram excluded an aneurysm of the conduit but no aortic root angiography was performed. On the next day, auscultation revealed a grade 3/6 to-and-fro murmur on the left sternal border. The echocardiogram showed appropriate pulmonary valve function and a diastolic color Doppler jet into the RVOT (Figure 1C and D), which was thought to terminate immediately below the Melody valve and was interpreted as the previously described VSD. Despite this, a normally functioning aortic valve and diastolic flow in the ascending aorta (AA) were documented. As the patient remained asymptomatic she was discharged home.
(A and B) Computed tomography showing the aortic end of the iatrogenic fistula near the right coronary ostium and the pulmonary end just proximal to the pulmonary valve; (C and D) transthoracic echocardiogram, parasternal long- and short-axis views of the iatrogenic fistula (*) between the aortic arch and the right ventricular outflow tract (RVOT); (E and F) aortic and conduit angiographies before percutaneous pulmonary valve implantation (PPVI); (G) conduit angiography after pre-stenting and after PPVI; (H) aortic angiography showing opacification of the RVOT.
During follow-up, the patient began to suffer from fatigue on moderate exertion. Transesophageal echocardiography and cardiac catheterization (Figure 1H and Video 1) showed a significant fistula between the AA and the RVOT with Qp:Qs of 2.1:1. Computed tomography (Figure 1A and B) further detailed the anatomy, with the aortic end of the fistula near the right coronary ostium and the pulmonary end just proximal to the valve. The patient underwent surgical repair of the communication with a direct double suture. Intraoperatively, a circular defect was found in the right sinus of Valsalva, 6 mm below the right coronary ostium and above the aortic annulus, with a diameter of 8 mm. The fistula opened in the RVOT in close proximity to the proximal end of the Melody valve. Six months later, the patient is asymptomatic and there is no evidence of the fistula on the echocardiogram. A small residual restrictive VSD (gradient 80 mmHg) has been documented since the postoperative period of the primary surgery.
DiscussionIatrogenic communications between the AA and the RVOT after PPVI are an uncommon complication. In the literature we found five patients who developed aortopulmonary communications after PPVI. Previous to PPVI, three of these patients had undergone a Ross procedure,2–4 one an arterial switch operation (ASO),1 and the other had a history of truncus arteriosus,1 the initial repair of which consisted of a single layer of autologous tissue between the AA and the PA. After PPVI they all developed a defect distal to the implanted valve. The reasons identified for its appearance were1,2,4: dilatation and calcification of the neoaorta; fragility and distortion of the neoaorta or PA anastomosis after a Ross procedure or an ASO, due to fibrosis developing between the aorta and the PA; and proximity of the aortic suture lines to the pulmonary valve. These create a weak point that can disrupt, due to mechanical effects, after PA angioplasty or PPVI. In the truncus arteriosus patient the existence of a single layer of tissue was the reason put forward.1
This case differs from the previous ones because the fistula was proximal to the implanted valve. We speculate that aggressive small conduit dilatation and/or the edges of the AndraStent struts may have caused the defect.
These fistulas may remain undiagnosed and grow over time. In the case presented, the lack of a post-PPVI aortic angiogram meant that the aorta-to-RVOT shunt was not observed. A high level of suspicion is therefore required for this unusual complication and, in order to diagnose it early, an aortic root angiogram should be routinely performed after PPVI.
ConclusionsIatrogenic aorta to RVOT fistula after PPVI is a rare and underdiagnosed complication. The etiology of these defects is still unclear, therefore a high level of suspicion is required and a post-PPVI aortic angiogram will help to diagnose this unusual complication earlier.
Conflicts of interestThe authors have no conflicts of interest to declare.