Takotsubo cardiomyopathy is an acute cardiac entity with clinical manifestations similar to myocardial infarction, accounting for 1-2% of acute coronary syndrome admissions. Its underlying pathophysiology is not yet well established. It is usually associated with acute physical or emotional stress, but the list of potential triggers has grown as the condition attracts the attention of the medical community. In order to diagnose the condition correctly and to gain new insights into it, we need to know its potential triggers as well as its clinical presentation and diagnostic criteria. We report a case of takotsubo cardiomyopathy triggered by hyponatremia.
A cardiomiopatia de Takotsubo é uma entidade nosológica que se caracteriza por manifestações clínicas similares às do enfarte agudo do miocárdio, representando cerca de 1-2% de todos os doentes admitidos por síndrome coronária aguda. A sua etiopatogenia ainda não está totalmente esclarecida. Geralmente, está associada a vigorosos estímulos físicos ou emocionais. Com o crescente reconhecimento desta entidade, a lista de potenciais estímulos despoletadores tem crescido nos últimos anos. O conhecimento destes novos estímulos permitirá não só um diagnóstico correcto mas também o aprofundamento dos seus mecanismos fisiopatológicos. Os autores descrevem um caso de cardiomiopatia de Takotsubo induzida por hiponatremia.
Takotsubo cardiomyopathy (TC) – also known as transient apical ballooning or stress cardiomyopathy – is a reversible clinical entity characterized by transient cardiac apical ballooning1. It constitutes an important differential diagnosis from acute myocardial infarction (MI) and probably accounts for 1-2% of all cases of suspected MI2. It typically affects post-menopausal women; cardiac enzyme elevation is minor and all morphologic and functional abnormalities evolve to full recovery in days to weeks. Coronary angiography (CA) is mandatory to establish the diagnosis in order to exclude obstructive coronary disease or evidence of acute plaque rupture. The importance of cardiac magnetic resonance imaging is growing because it can distinguish myocarditis and coronary microvascular ischemic disease, two important diagnoses in the differential work-up of TC3. The precise pathophysiology is mostly unknown. One of the most widely accepted theories states that in response to emotional or physical distress, increased sympathetic neuronal and adrenomedullary hormonal outflow results in toxic high circulating catecholamine levels4. Although the typical clinical descriptions and the pathophysiological mechanisms point to physical or emotional distress as the precipitating factor, retrospective studies show that some of these patients have no identifiable preceding stressor. Many triggers have been described since this entity gained recognition. We describe a case of TC that was triggered by severe hyponatremia.
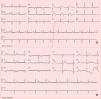
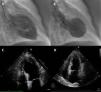
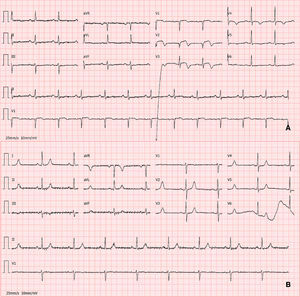
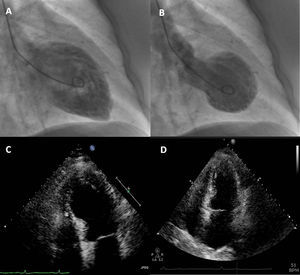
Case reportA 74-year-old woman was admitted to our emergency room with acute chest pain of three hours’ duration that woke her from sleep. Her medical history included dyslipidemia, hypertension and diabetes. Her usual medications included metformin, fluvastatin, perindopril and indapamide. She denied previous angina-like symptoms but reported mild nausea in the previous month. Her physical examination was unremarkable. The first electrocardiogram (ECG) showed sinus rhythm with QS waves in precordial leads V1-2, 2-mm ST-segment elevation and T-wave inversion in precordial leads V1-4 (Fig. 1A). On admission, cardiac biomarkers were mildly elevated with troponin T of 0.14 ng/ml (normal range 0.0-0.06 ng/ml) and CK-MB of 5.9 ng/ml (normal range 0.6-3.5 ng/ml). She was presumed to have ST-elevation MI and was emergently referred for CA, which revealed no coronary stenosis. Ventriculography showed akinesia of the anterior wall and apex with ejection fraction (EF) of 23% (Fig. 2A-B). Transthoracic echocardiography revealed preserved contractility of basal segments and akinesia of all the others with apical ballooning morphology (Fig. 2C-D). Subsequent blood analysis disclosed serum sodium of 118 mmol/l (normal 135-145 mmol/l), all other tests being within normal limits. Etiologic evaluation of the hyponatremia led to the conclusion that it was due to indapamide therapy for hypertension, after exclusion of several other causes such as hypothyroidism and adrenal insufficiency. After withdrawal of indapamide and isotonic saline fluid perfusion, her sodium increased to 139 mmol/l by the end of the first week. Pheochromocytoma was subsequently ruled out by normal catecholamine levels in a 24-hour urine sample. Inflammatory markers including C-reactive protein remained normal, making a diagnosis of myocarditis unlikely. The patient became asymptomatic in the first 24hours of hospital stay and there were no further complications. She was discharged after two weeks. At that time, echocardiography demonstrated full recovery of EF (Fig. 2D) and the ECG showed resolution of the abnormalities described (Fig. 1B). She began aspirin and beta-blocker therapy and suspended indapamide. At 2-month follow-up, the patient was asymptomatic and with normal ventricular systolic function.
Contrast left ventriculography: diastolic (A) and systolic (B) frames showing motion abnormalities of the mid and apical segments. Transthoracic echocardiogram in 4-chamber apical view (systolic frames) revealing akinesia of mid-apical segments and apical ballooning (C), no longer present at discharge (D).
Hyponatremia is the most common electrolyte disorder in hospitalized patients but it is usually mild and clinically irrelevant. Finding the etiology of hyponatremia can be challenging since there are many conditions associated with it5. In the case presented, diagnostic work-up pointed to indapamide-induced hyponatremia. Several case reports have established a causal relationship between this diuretic agent and severe hyponatremia6,7. Its presentation is dominated by neurological symptoms due to brain swelling, cardiac manifestations being uncommon.
There are a few reports of hyponatremia-induced takotsubo-like cardiomyopathy. However, some of them presented significant confounding factors such as seizures, adrenal insufficiency and hypothyroidism8–10. To our knowledge, our case is the third description of TC triggered by isolated severe hyponatremia. Any confounding factor was ruled out, enabling us to assume a causal relationship between the two entities. AbouEzzeddine and Prasad11 described two patients with hyponatremia, one related to diuretic use and the other to inappropriate antidiuretic hormone secretion, both presenting this reversible cardiomyopathy. Considering the most widely accepted theories on the pathophysiological mechanisms of TC, we suggest that central nervous system dysfunction induced by hyponatremia can result in myocardial injury due to elevated catecholamine levels. On the other hand, we speculate that hyponatremia could interfere with myocardial inotropism by modulation of cardiomyocyte Na+/Ca2+ exchange and also through myocardial swelling associated with hypotonicity12.
The case presented demonstrates that severe hyponatremia can be regarded as a potential trigger of TC. In an appropriate clinical setting, knowledge of newly recognized precipitating factors is invaluable when considering this diagnosis. It also illustrates how a thorough clinical evaluation can be the key to reveal new and unsuspected pathophysiological mechanisms in this not fully understood entity.
Conflicts of interestThe authors declare they have no conflicts of interest.