Levosimendan infusion in the outpatient setting improves the clinical status of heart failure (HF) patients, although its hemodynamic effects are not entirely known. Remote monitoring using the CardioMEMS™ system enables daily assessment of pulmonary artery pressure (PAP) and estimation of cardiac output (CO). We aimed to assess the hemodynamic effects of outpatient levosimendan infusion using CardioMEMS™.
MethodsAll patients admitted for 6-hour levosimendan infusion (performed every 14 days) and using the CardioMEMS™ remote monitoring system were included in a prospective single-center registry. Clinical and laboratory data were recorded. Systolic, diastolic, and mean PAP, heart rate, CO, and stroke volume (SV) were assessed daily.
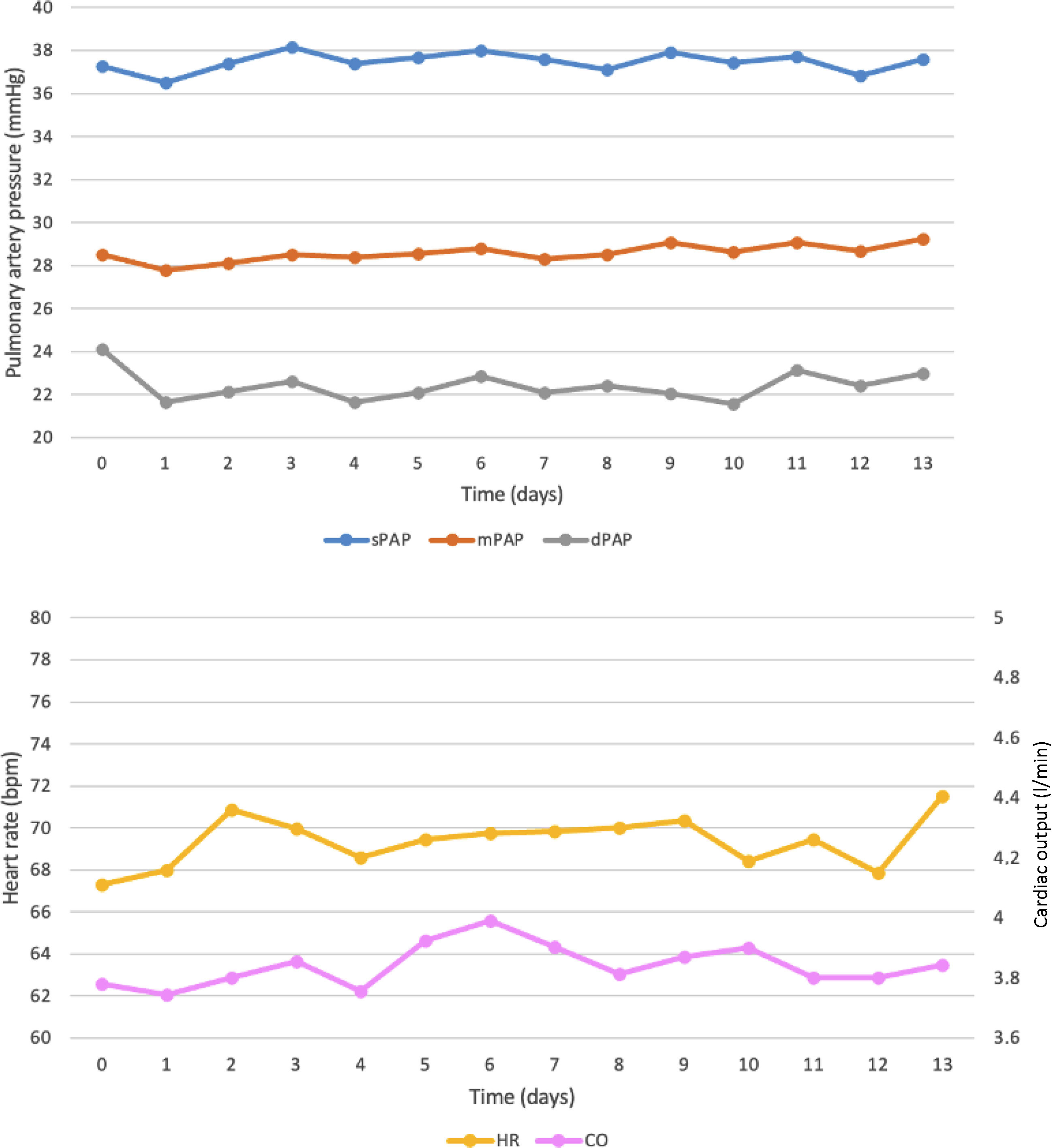
ResultsA total of 25 sessions were performed in three patients. There were no adverse events or significant therapy adjustments. There was a significant reduction in diastolic PAP the day after levosimendan infusion compared to baseline (day prior to infusion) (24.1±4.1 mmHg vs. 21.6±2.9 mmHg, p=0.006). Thereafter, diastolic PAP stabilized and remained significantly lower than baseline up to day 10. There were no significant differences in systolic PAP, mean PAP, heart rate, CO, or SV at any timepoint analyzed, although there was a nonsignificant increase in CO with a peak at day 6.
ConclusionOutpatient levosimendan infusion was associated with an early reduction in diastolic PAP, which was maintained for 10 days. The CardioMEMS™ system may enable a better understanding of outpatient hemodynamics in advanced HF. To our knowledge, there are no published data on this subject.
A perfusão de levosimendan em regime de ambulatório melhora o quadro clínico de doentes com insuficiência cardíaca (IC), embora os seus efeitos hemodinâmicos não sejam totalmente conhecidos. A monitorização remota usando o sistema CardioMEMS™ permite a avaliação diária da pressão arterial pulmonar (PAP) e a estimativa do débito cardíaco (DC). O nosso objetivo foi avaliar os efeitos hemodinâmicos da perfusão em ambulatório de levosimendan usando o CardioMEMS™.
MétodosTodos os doentes admitidos em Hospital de Dia para perfusão de levosimendan durante 6 horas (realizada a cada 14 dias) e utilizando concomitantemente o sistema de monitorização remota CardioMEMS™ foram incluídos num registo prospectivo unicêntrico. Os dados clínicos e laboratoriais foram registados. A PAP sistólica, diastólica e média, frequência cardíaca, DC e volume sistólico (VS) foram avaliados diariamente.
ResultadosForam realizadas 25 sessões em 3 doentes. Não houve eventos adversos nem ajustes terapêuticos significativos. Verificou-se uma redução significativa na PAP diastólica no dia seguinte à perfusão de levosimendan comparativamente com o valor basal (dia anterior à perfusão) (24,1±4.1mmHg versus 21,6±2,9mmHg, p=0,006). Posteriormente, a PAP diastólica estabilizou e manteve-se significativamente mais baixa em comparação com o valor basal até ao dia 10. Não houve diferenças significativas na PAP sistólica, PAP média, frequência cardíaca, DC ou VS em qualquer ponto de tempo analisado, embora tenha havido um aumento não significativo no DC com pico ao sexto dia.
ConclusãoA perfusão em ambulatório de levosimendan associou-se à redução precoce da PAP diastólica, que se manteve por 10 dias. O CardioMEMS™ pode permitir uma melhor compreensão da hemodinâmica em ambulatório na IC avançada. Até onde sabemos, não existem dados publicados sobre este assunto.
Heart failure (HF) with reduced ejection fraction (HFrEF) is associated with high morbidity and mortality despite the use of evidence-based optimal medical and device therapy.1 This is particularly true in patients with advanced HF, who may benefit from specific treatments, such as heart transplantation, assist devices or palliative care.2 Aside from the use of such therapeutic options, a 24-hour infusion of levosimendan has been used to transiently improve the clinical status of patients with advanced HF.3–6 Moreover, levosimendan has been used in protocols of adjusted (reduced) dose and repeated infusions in the outpatient setting with encouraging results,7–9 as supported by data from the LION-HEART and LAICA trials.10,11
Levosimendan is an inotropic drug with three mechanisms of action: positive inotropy, vasodilation, and cardioprotection.12–16 Its pharmacokinetics means that its metabolite OR1896 has a longer duration of action than other catecholaminergic inotropes.17 It is well known that a 24-hour levosimendan infusion in patients hospitalized for HF improves their hemodynamic status.3,4,6 However, there are no available data on the hemodynamic effects of adjusted-dose levosimendan infusion in the outpatient context, since the assessment of hemodynamic data in this setting is hampered by logistic constraints. Moreover, the hemodynamic effects of adjusted-dose levosimendan infusion in the outpatient setting may differ from the effects of a 24-hour full-dose infusion and from its effects in acute decompensated HF. The availability of daily hemodynamic data after outpatient levosimendan infusion could contribute to a better understanding of the mechanisms that lead to clinical improvement following the infusions and possibly to optimization of the infusion protocol.
Remote invasive monitoring using the CardioMEMS™ system (Abbott Scientific, Atlanta, GA, USA) enables daily assessment of pulmonary artery pressure (PAP) and estimation of cardiac output (CO).18 It is currently approved for chronic HF patients in New York Heart Association (NYHA) functional class III and with at least one prior hospitalization for HF within the previous year, regardless of ejection fraction.1 Of note, PAP measurements using CardioMEMS™ show a strong correlation with PAP obtained using a Swan-Ganz catheter.19 The CardioMEMS™ system may thus enable accurate daily hemodynamic assessment of therapeutic interventions in the outpatient setting.
ObjectivesWe aimed to assess the hemodynamic effects of levosimendan infusion in the outpatient setting using the CardioMEMS™ system. To the best of our knowledge, there are no published data on this subject.
MethodsEthicsThe investigation conforms to the principles outlined in the Declaration of Helsinki. The institutional ethics committee approved the study protocol (CHULC ethics committee approval no. 1031/2021) and all patients provided written informed consent.
Study design and patients includedWe performed a prospective single-center analysis of HF patients referred for ambulatory levosimendan therapy and with active remote monitoring based on the CardioMEMS™ system.
Patients were considered eligible if they met the following criteria: HF, irrespective of left ventricular ejection fraction (LVEF); NYHA functional class ≥II; under guideline-directed medical therapy for at least three months; referral for levosimendan infusions in the outpatient setting by the HF team due to refractory symptoms; and under active remote monitoring based on the CardioMEMS™ system.
Exclusion criteria were as follows: any intervention for coronary artery disease, valvular disease or atrial fibrillation in the three months prior to recruitment; placement of cardiac implantable electronic devices in the three months prior to recruitment; planned change in HF medical therapy, device therapy, or percutaneous or surgical intervention; inclusion in the waiting list for heart transplantation or left ventricular assist device (LVAD); hemodynamic instability with systolic blood pressure ≤90 mmHg; hypersensitivity to levosimendan or any excipient used; history of torsades de pointes; left ventricular outflow tract obstruction; hypertrophic/restrictive cardiomyopathy; estimated glomerular filtration rate <30 ml/min/m2; severe liver disease; or patient refusal or inability to attend the levosimendan infusion sessions. Of note, although listing for an LVAD or cardiac transplantation was an exclusion criterion for CardioMEMS™ implantation, if there was a need for such therapies after CardioMEMS™ implantation and the patient was started on levosimendan, they were not excluded from the study. Regarding HF medical therapy, no planned changes in prognosis-modifying treatment were made at the time of patient inclusion. Regarding diuretic therapy, patients were required to have a stable congestion level at the time of inclusion. However, adjustments in diuretic doses were expected during the study and were made according to the guidance provided by the CardioMEMS™ device.
Baseline and follow-up assessmentBaseline clinical, demographic, laboratory, electrocardiographic, and echocardiographic data were collected at study inclusion. Clinical and laboratory data were collected at each levosimendan session. Systolic, diastolic, and mean PAP, heart rate (HR), CO and stroke volume (SV) were assessed daily using the CardioMEMS™ system. Hemodynamic data were recorded from seven days prior to the initial infusion and following the final infusion.
Outpatient levosimendan infusion protocolOur outpatient levosimendan infusion protocol has been described previously.20,21 Levosimendan infusion was started at a dose of 0.1 mg/kg/min and titrated to 0.2 mg/kg/min after the first two hours at the first infusion. If well tolerated, following doses were at the rate of 0.2 mg/kg/min. Each session lasted for six hours and was performed every 14 days. Cardioprotective medication for HF was to be maintained during the day of the procedure. The diuretic dose on the day of the treatment was halved or discontinued by clinical decision. If venous blood gas analysis documented a potassium level below 3.5 mmol/l, treatment was rescheduled, and potassium was replaced until the following session.
Remote invasive monitoring using the CardioMEMS™ systemThe CardioMEMS™ sensor was implanted in the pulmonary artery according to the manufacturer's instructions22 and calibrated with right heart catheterization (RHC) using a balloon wedge-pressure catheter and indirect Fick CO assessment. Patients performed daily readings of hemodynamic data using the portable antenna and the data were transmitted wirelessly to a digital database that was accessible to clinicians for regular review.
The CardioMEMS™ system measures PAP and HR. CO is estimated using a proprietary algorithm (Abbott Scientific, Atlanta, GA, USA) based on the PAP waveform, mean PAP, HR, and a reference CO measured at implantation.23 Although estimated CO is not yet approved for routine use in clinical practice, the algorithm has been developed, refined, and retrospectively tested against clinical RHC data, demonstrating noninferiority to currently used CO measurement methods.24
EndpointsThe main study endpoints were changes in systolic, diastolic, and mean PAP and CO following levosimendan infusion.
Statistical analysisCategorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviation, or medians and interquartile range for variables with skewed distribution. Normal distribution was checked using the Shapiro–Wilk test. Variables of interest were compared before and after each levosimendan infusion using the paired Student's t test or the Wilcoxon signed-rank test, as appropriate. Two-sided p-values <0.05 were considered statistically significant. Data were analyzed using SPSS version 25 (IBM).
ResultsA total of 25 sessions were carried out in three patients (six sessions in patient 1, 17 in patient 2, and two in patient 3). All patients reached and tolerated the 0.2 mg/kg/min levosimendan infusion dose, without the need for dose reduction, throughout all sessions.
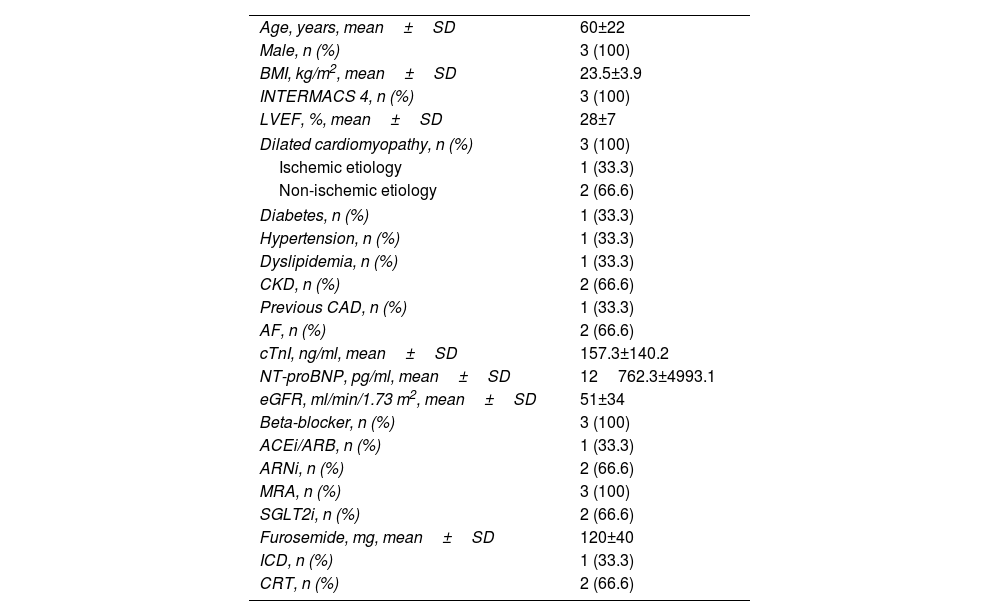
Clinical and demographic dataBaseline characteristics are summarized in Table 1. Mean age was 60±22 years, all patients were male, all were in Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) level 4, mean LVEF was 28±7% and mean N-terminal brain natriuretic peptide (NT-proBNP) was 12762.3±4993.1 pg/ml.
Baseline characteristics of the study population.
| Age, years, mean±SD | 60±22 |
| Male, n (%) | 3 (100) |
| BMI, kg/m2, mean±SD | 23.5±3.9 |
| INTERMACS 4, n (%) | 3 (100) |
| LVEF, %, mean±SD | 28±7 |
| Dilated cardiomyopathy, n (%) | 3 (100) |
| Ischemic etiology | 1 (33.3) |
| Non-ischemic etiology | 2 (66.6) |
| Diabetes, n (%) | 1 (33.3) |
| Hypertension, n (%) | 1 (33.3) |
| Dyslipidemia, n (%) | 1 (33.3) |
| CKD, n (%) | 2 (66.6) |
| Previous CAD, n (%) | 1 (33.3) |
| AF, n (%) | 2 (66.6) |
| cTnI, ng/ml, mean±SD | 157.3±140.2 |
| NT-proBNP, pg/ml, mean±SD | 12762.3±4993.1 |
| eGFR, ml/min/1.73 m2, mean±SD | 51±34 |
| Beta-blocker, n (%) | 3 (100) |
| ACEi/ARB, n (%) | 1 (33.3) |
| ARNi, n (%) | 2 (66.6) |
| MRA, n (%) | 3 (100) |
| SGLT2i, n (%) | 2 (66.6) |
| Furosemide, mg, mean±SD | 120±40 |
| ICD, n (%) | 1 (33.3) |
| CRT, n (%) | 2 (66.6) |
ACEi/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AF: atrial fibrillation; ARNi: angiotensin receptor/neprilysin inhibitor; BMI: body mass index; CAD: coronary artery disease; CKD: chronic kidney disease; CRT: cardiac resynchronization therapy; cTnI: cardiac troponin I; ICD: implantable cardioverter-defibrillator; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SGLT2i: sodium-glucose cotransporter type 2 inhibitor.
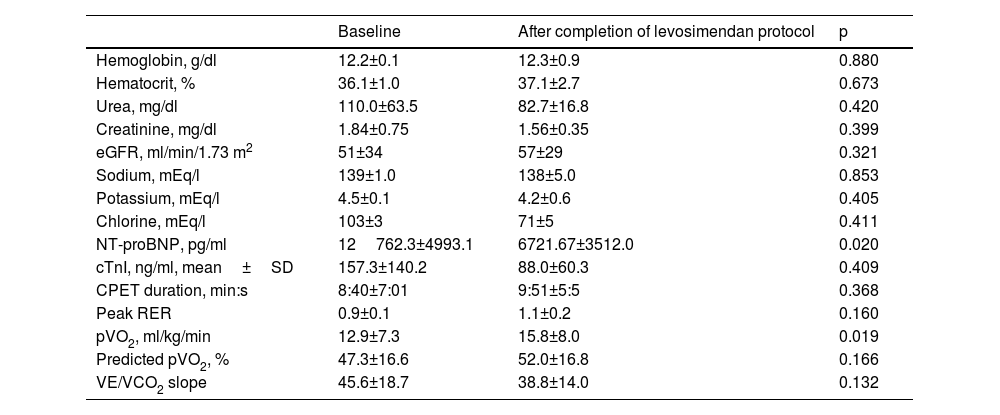
There were no infusion-related adverse events, HF decompensations or changes in neurohormonal modulatory therapy during the study period. Also, during this period, diuretic doses were not significantly reduced and there was no need to reschedule levosimendan sessions because of potassium levels. Table 2 presents laboratory and cardiopulmonary exercise test (CPET) data at baseline (study entry) and at the end of the levosimendan protocol in the three patients. There was an improvement in NYHA class in two patients (class III to class II), while patient 3 remained in class II. Regarding clinical outcomes, the patients remained stable, with no major cardiac events to date, including hospitalizations for cardiac or noncardiac causes. No deaths were recorded. Levosimendan administration was associated with an improvement in NT-proBNP levels, which showed a statistically significant reduction after the infusions. Additionally, peak oxygen consumption (pVO2) increased significantly, indicating enhanced exercise capacity. Other baseline characteristics and CPET parameters showed trends of improvement, though these were not statistically significant.
Laboratory and cardiopulmonary exercise test parameters before and after levosimendan sessions.
| Baseline | After completion of levosimendan protocol | p | |
|---|---|---|---|
| Hemoglobin, g/dl | 12.2±0.1 | 12.3±0.9 | 0.880 |
| Hematocrit, % | 36.1±1.0 | 37.1±2.7 | 0.673 |
| Urea, mg/dl | 110.0±63.5 | 82.7±16.8 | 0.420 |
| Creatinine, mg/dl | 1.84±0.75 | 1.56±0.35 | 0.399 |
| eGFR, ml/min/1.73 m2 | 51±34 | 57±29 | 0.321 |
| Sodium, mEq/l | 139±1.0 | 138±5.0 | 0.853 |
| Potassium, mEq/l | 4.5±0.1 | 4.2±0.6 | 0.405 |
| Chlorine, mEq/l | 103±3 | 71±5 | 0.411 |
| NT-proBNP, pg/ml | 12762.3±4993.1 | 6721.67±3512.0 | 0.020 |
| cTnI, ng/ml, mean±SD | 157.3±140.2 | 88.0±60.3 | 0.409 |
| CPET duration, min:s | 8:40±7:01 | 9:51±5:5 | 0.368 |
| Peak RER | 0.9±0.1 | 1.1±0.2 | 0.160 |
| pVO2, ml/kg/min | 12.9±7.3 | 15.8±8.0 | 0.019 |
| Predicted pVO2, % | 47.3±16.6 | 52.0±16.8 | 0.166 |
| VE/VCO2 slope | 45.6±18.7 | 38.8±14.0 | 0.132 |
CPET: cardiopulmonary exercise test; pVO2: peak oxygen consumption; RER: respiratory exchange ratio; SD: standard deviation; VE/VCO2: minute ventilation/carbon dioxide production.
Results are presented as mean±standard deviation.
There were no transmission failures of the CardioMEMS™ system and reading compliance was 95% during the study period.
Figure 1 presents systolic, mean and diastolic PAP, CO, and HR before and after each levosimendan infusion. There was an early reduction in diastolic PAP on the first day following levosimendan infusion compared to the day prior to the infusion (baseline) (24.1±4.1 mmHg vs. 21.6±2.9 mmHg, p=0.006). Thereafter, diastolic PAP remained stable and significantly lower than baseline up to day 10 after the infusion. There were no significant differences in systolic or mean PAP before and after levosimendan infusion at any timepoint analyzed or significant changes in CO, HR, or SV after levosimendan infusion compared to baseline. There was a nonsignificant increase in CO compared to baseline with a peak at day 6 after the infusion (4.0 l/min vs. 3.8 l/min, p=0.105), followed by a decline in CO in the following days.
Hemodynamic data before and up to day 13 after six-hour levosimendan infusion. CO: cardiac output; dPAP: diastolic pulmonary artery pressure; HR: heart rate; mPAP: mean pulmonary artery pressure; sPAP: systolic pulmonary artery pressure. 0 (zero) corresponds to the CardioMEMS™ measurement on the day before the infusion.
To our knowledge, this is the first study assessing the day-to-day hemodynamic effects of levosimendan infusion in the outpatient setting using the CardioMEMS™ system. In 25 sessions of levosimendan infusion performed in three patients with advanced HF, the main finding was a significant early reduction in diastolic PAP after each infusion, an effect that was maintained up to 10 days. This may provide insights into the mechanisms underlying clinical improvement following levosimendan infusion and supports the time intervals between infusions used in the protocol.
Levosimendan is an inotropic drug that interacts with cardiac troponin C, which increases the calcium sensitivity of myocardial contractile units, thus increasing cardiac contractility and enhancing rapid ventricular filling,13 without increasing myocardial oxygen consumption.12 Additionally, it promotes opening of adenosine triphosphate-dependent potassium (KATP) channels on vascular smooth muscle cells, which results in vasodilatation, causing a reduction in right ventricular preload and afterload.14,15 The effect of levosimendan in opening KATP channels in the mitochondrial inner membrane can reduce the production of free radicals within the cells, conferring cardioprotection.12,16 The effects of levosimendan and its metabolite OR1896 reportedly last up to seven days.17
Previous studies have reported that the maximum effect of 24-hour infusion of levosimendan on pulmonary capillary wedge pressure (PCWP) and CO in patients with HF occurred within 24 hours4–6 and the estimated duration of the decrease in PCWP was 7–9 days, while the increase in CO lasted for 12–13 days.25 Nieminen et al.4 assessed the hemodynamic effects of levosimendan in hospitalized patients with NYHA class II–IV stable HF of ischemic etiology. A 10-min intravenous bolus of three, six, 12, 24 or 36 μg/kg was followed by a 24-hour infusion of 0.05, 0.1, 0.2, 0.4 or 0.6 μg/kg/min, respectively. Hemodynamic parameters were measured using a Swan-Ganz catheter. A clear and consistent relationship was observed between the levosimendan dose and hemodynamic responses. At 24 hours, all doses produced significant decreases in PCWP, and infusions of 0.4 and 0.6 μg/kg/min produced significant increases in CO. Significant linear reductions in mean PAP, systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) were also demonstrated. In patients with decompensated HF, Moertl et al.5 showed that levosimendan infusion over 24 hours was associated with an increase in CO and decreases in PCWP, PAP and SVR with sustained effects after 48 hours as assessed by a Swan-Ganz catheter. Also, in patients with decompensated HF, Slawsky et al.6 showed short-term effects (up to six hours) of levosimendan in hemodynamic parameters (measured by a pulmonary artery catheter): an hourly bolus of 6 μg/kg, followed by a continuous infusion initially at a rate of 0.1 μg/kg/min uptitrated to a maximum rate of 0.4 μg/kg/min led to decreases in mean PCWP, mean right atrial pressure, mean PAP, SVR and PVR. Cardiac index and SV increased at all infusion rates.
In addition to inpatient use, levosimendan has also been used in an outpatient setting, which is supported by data from the LION-HEART and LAICA trials.10,11 These trials of intermittent levosimendan infusion demonstrated significant improvements in symptoms, quality of life, HF hospitalizations, and HF-related mortality, along with improvement in surrogate markers, such as levels of cardiac biomarkers. However, the hemodynamic effects of intermittent levosimendan infusions in the outpatient setting are not known. Indeed, the hemodynamic effects of levosimendan using such protocols may theoretically differ from those previously described of a full-dose 24-hour infusion and/or in-hospital levosimendan infusion during HF decompensation.
It is recognized that levosimendan's effectiveness may vary across different severities of HF and be reduced in more advanced stages according to the INTERMACS scale, as indicated by previous studies and clinical experience. Moreover, while we are aware that HFpEF patients were not included in the LION-HEART and LAICA trials, in our center CardioMEMS™ is implanted regardless of ejection fraction, in accordance with the results of clinical trials.26–28 Including HF with preserved ejection fraction (HFpEF) patients could have provided valuable insights into the broader applicability of levosimendan therapy across different HF subtypes, even though we anticipated recruiting few HFpEF patients. In fact, no patients with HFpEF met the inclusion criteria and therefore our findings are only applicable to patients with HFrEF.
The results of this study are in line with those reported with 24-hour infusion protocols regarding the significant early reduction in diastolic PAP. Our results point to a later peak in CO, which may occur in the following days. Regarding the duration of the effects, the reduction in diastolic PAP was sustained up to day 10 after the infusion, which is consistent with the reported duration of the effect on PCWP of 24-hour levosimendan infusion. Of note, the observed effect of levosimendan on diastolic PAP supports the scheduled period of 14 days between infusions.
Also of note, the main hemodynamic effect of levosimendan was observed in diastolic PAP, which is clinically significant. Diastolic PAP is an appropriate surrogate for PCWP and left atrial pressure.29 Measuring PCWP reveals the cumulative hemodynamic impact of ventriculoatrial coupling and its operating compliance on the pulmonary circulation, on the basis of which the dosage of diuretic drugs to reduce pulmonary venous and capillary pressure can be titrated.25,30 Baseline diastolic PAP can predict HF events28 and changes in PAP can be used to predict both HF hospitalization and mortality.31,32 Diastolic PAP is the target parameter of remote monitoring of HF using the CardioMEMS™ system in most centers.32 The reduction of diastolic PAP with levosimendan infusion may explain the early symptomatic improvement after infusion, even without a significant increase in CO, in the absence of changes in diuretic or vasodilator therapies.
It should be borne in mind that interpreting clinical benefits in more advanced HF patients is complex and multifactorial. The primary objective of this study was to assess the hemodynamic effects of outpatient levosimendan infusion irrespective of their role in clinical improvement. Regardless, the relatively modest reduction in diastolic PAP may have contributed to clinical improvement, although other factors may also be involved, such as the benefit of periodic inotrope infusions and their relation to the frequent reassessment of these very severe patients.
The CardioMEMS™ system is approved for use in patients with HF to reduce the risk of hospitalization, and has also been extremely useful for a better understanding of the pathophysiology of HF.1,18,32 By providing daily assessment of hemodynamic parameters, including PAP and CO, it enables continuous characterization of the effects of therapeutic interventions in HF over time.33 Of note, PAP pressures assessed in the hospital setting, including those measured by RHC, are significantly different from those obtained in the outpatient setting.34 PAP values are known to rise in the days following hospital discharge.34 This discrepancy may be due to fasting before invasive procedures, different compositions of meals, including lower salt content in hospital meals, and greater adherence to medical therapy during hospitalization.34 The CardioMEMS™ system may therefore offer a more representative and clinically meaningful characterization of the hemodynamic effects of levosimendan infusion than other invasive methods performed in the hospital setting.
LimitationsThere are limitations in our study that should be acknowledged. The sample size was small, consisting of 25 levosimendan infusions in three patients, though most of the sessions (17 out of 25) were based on data from a single patient. This study design allowed us to detect significant changes in PAP. However, the limited number of patients restricts the robustness of our conclusions and explains the lack of variation in variables such as systolic PAP, mean PAP, CO, and SV. Moreover, this was a single-center study, and results from larger, multicenter cohorts are needed to confirm our results. In addition, patients received predefined doses of levosimendan without uptitration. This fulfills the requirements of a dose–response study but does not represent the clinical situation in which therapy would normally be initiated at low doses and titrated until the desired effects were achieved.
ConclusionOutpatient six-hour levosimendan infusions were associated with an early reduction in diastolic PAP (a surrogate of left filling pressures) that lasted for 10 days. The CardioMEMS™ system may enable a better understanding of outpatient hemodynamics in advanced HF.
FundingThis research has received no external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
We gratefully acknowledge all the study participants and patients.