Cognitive impairment, anxiety and depression are common in heart failure (HF) patients and its evolution is not fully understood.
ObjectivesTo assess the cognitive status of HF patients over time, its relation to anxiety and depression, and its prognostic impact.
MethodsProspective, longitudinal, single center study including patients enrolled in a structured program for follow-up after hospital admission for HF decompensation. Cognitive function, anxiety/depression state, HF-related quality of life (QoL) were assessed before discharge and during follow-up (between 6th and 12th month) using Montreal Cognitive Assessment (MoCA), Hospital Anxiety and Depression Scale (HADS) and Kansas City Cardiomyopathy Questionnaire, respectively. HF related outcomes were all cause readmissions, HF readmissions and the composite endpoint of all-cause readmissions or death.
Results43 patients included (67±11.3 years, 69% male); followed-up for 8.2±2.1 months. 25.6% had an abnormal MoCA score that remained stable during follow-up (22.6±4.2 vs. 22.2±5.5; p=NS). MoCA score <22 at discharge conferred a sixfold greater risk of HF readmission [HR=6.42 (1.26-32.61); p=0.025], also predicting all-cause readmissions [HR=4.00 (1.15-13.95); p=0.03] and death or all-cause readmissions [HR=4.63 (1.37-15.67); p=0.014]. Patients with higher MoCA score showed a greater ability to deal with their disease (p=0.038). At discharge, 14% and 18.6% had an abnormal HADS score for depression and anxiety, respectively, which remained stable during follow-up and was not related to MoCA.
ConclusionsCognitive function, anxiety and depressive status remain stable in HF patients despite optimized HF therapy. Cognitive status shall be routinely screened to adopt attitudes that improve management as it has an impact on HF-related QoL and prognosis.
A disfunção cognitiva, ansiedade e depressão são comuns nos doentes com insuficiência cardíaca (IC) e a sua evolução não é integralmente conhecida.
ObjetivosAvaliar o estado cognitivo dos doentes com IC ao longo do tempo, a sua relação com ansiedade/depressão e o seu impacto prognóstico.
MétodosEstudo prospetivo, longitudinal, unicêntrico de doentes seguidos num programa estruturado após internamento por IC descompensada. A função cognitiva, estado ansioso/depressivo, qualidade de vida (QV) relacionada com a IC foram avaliadas antes da alta e durante o seguimento (entre 6.°-12.° mês) utilizando o Montreal Cognitive Assessment (MoCA), a Hospital Anxiety and Depression Scale (HADS) e o Kansas City Cardiomyopathy Questionnaire (KCCQ), respetivamente. Avaliaram-se outcomes relacionados com IC: reinternamento por qualquer causa, reinternamentos por IC e objetivo composto de morte/reinternamento.
ResultadosForam incluídos 43 doentes (67±11,3 anos, 69% homens); seguidos por 8,2±2,1 meses. 25,6% tinham um resultado anormal no MoCA que se manteve estável no seguimento (22,6±4,2 versus 22,2±5,5; p=NS). Um resultado do MoCA<22 na alta conferiu um risco seis vezes superior de reinternamento por IC[HR=6,42 (1,26-32,61); p=0,025], sendo também preditor de reinternamento por qualquer causa [HR=4,00 (1,15-13,95); p=0,03] e do objetivo composto [HR=4,63 (1,37-15,67); p=0,014]. Doentes com um resultado mais elevado no MoCA apresentaram maior capacidade para lidar com a IC (p=0,038). Na alta, 14% e 18,6% tinham um resultado anormal no HADS para a depressão e ansiedade, respetivamente, que se manteve estável no seguimento e não se associou ao MoCA.
ConclusõesO funcionamento cognitivo e o estado ansioso/depressivo mantêm-se estáveis apesar da terapêutica optimizada para a IC. Tendo impacto na QV relacionada com a IC e no prognóstico, a função cognitiva deverá ser rastreada por rotina para adotar atitudes que melhorem a abordagem a estes doentes.
Heart failure (HF) is a major public health problem affecting globally 1-2% of the adult population in developed countries, and more than 10% of those >70 years-old.1–3 Despite the advances in therapy in recent decades, HF is associated with high mortality and morbidity, especially recurrent hospitalizations, and reduced functional status and quality of life (QoL).1,4 Given the aging population, the overall prevalence of the syndrome is expected to rise in the near future.3,4
Heart failure is a complex syndrome, not limited to the heart itself, but involving whole body systems, including the central nervous system. Brain-heart involvement is believed to be bidirectional and diverse interactions (some still misunderstood), between cardiac and cerebral function may lead to cognitive impairment, anxiety and depression,5,6 which in turn can contribute to further deterioration of HF. Cognitive function is essential to understand information, to learn and recall specific knowledge, and these skills are used to solve problems and plan actions in the challenges posed by everyday life. Anxiety and depression are also common in association with HF and contribute to morbidity and increased healthcare use.5,7 Anxiety and depression seems to contribute to abnormal cognitive status8 in patients with other chronic diseases,9 however the simultaneous assessment of anxiety, depression and cognitive impairment (CI) in HF patients was poorly studied.10–12
The high prevalence of cognitive impairment in HF patients is already recognized,5,13 ranging from 25% to 75%,5,13 but there is limited knowledge of the evolution of cognitive status over time in these patients.14 In addition, some of the published longitudinal studies include patients enrolled more than 10 years ago,14–19 prior to significant changes in HF treatment strategies. Furthermore, some studies reveal contradictory results in what concerns cognitive status over time.13,20 Those performed by Riegel et al.,21 Alosco et al.22 and Stanek et al.,19 who studied HF patients’ cognition with a battery of neuropsychological tests, found both stability and/or improvement in cognitive testing over time; however other authors23 reported decreased cognitive function over time in HF patients.
The aim of this study was to assess, in a population HF followed accordingly to a structured HF program and under optimized therapy, global cognitive status over time, its association with anxiety and/or a depressive state, and to assess the prognostic impact of this condition on HF outcomes (all-cause readmissions, HF readmissions and the composite endpoint of death or readmission). To our knowledge, this is the first study with longitudinal data that simultaneously investigated cognitive status, anxiety and depression in Portuguese patients with HF followed-up in accordance with optimal recommended practices.1
MethodsSetting, study design and patient selection criteriaA prospective, single center study conducted at a tertiary hospital (Cardiology Department, Hospital Universitário de Santa Maria, Lisbon, Portugal).
All adult patients (≥18 years-old), consecutively admitted (index-admission) to the cardiology ward with acute HF (de novo or chronic decompensated HF), defined according to criteria in the Guidelines of the European Society of Cardiology (ESC),1 were potentially eligible for inclusion in a post-discharge follow-up HF-program (by protocol), after written informed consent was obtained. This structured post-discharge follow-up program has already been described elsewhere.24 Specific and validated questionnaires for assessment of QoL, and evaluation of cognitive status and depression and chronic anxiety states by application of specific and validated questionnaires at pre-specified times, are components of the program. Patients with language barriers (unable to speak and/or understand Portuguese), visual and auditory deficits, and previously diagnosed neurological or psychiatric disease were excluded from the present study.
Screening proceduresCognitive status, chronic anxiety and depression, were screened at hospital discharge, when the patients were stable, to reduce a possible confounder related to disease decompensation and hospitalization, and between the 6th and the 12th month after discharge.
The most recent ESC Guidelines for the diagnosis and treatment of acute and chronic HF1 stated that cognitive function can be assessed using the Mini-Mental State Examination (MMSE)25 or the Montreal Cognitive Assessment (MoCA).26 In this study the validated Portuguese version of the MoCA was used. It is a cognitive screening instrument with greater sensitivity than the MMSE to milder stages of cognitive decline.31 It is a one-page test with a maximum score of 30 points, which defines CI according to a score of <22 (cut-off validated for the Portuguese population),28,29 assessing eight cognitive domains: executive functions; visuospatial abilities; short-term memory; language; attention, concentration, and working memory; temporal and spatial orientation.26,30
The MoCA score was assessed as a continuous variable to compare evolution over time and as a dichotomous variable based on a cutoff score of 22 to distinguish patients with a normal versus an abnormal score.
Chronic anxiety and depression were assessed using the validated Portuguese version of the Hospital Anxiety and Depression Scale (HADS).27 The HADS is composed of seven questions relevant to either generalized anxiety or depression, with each item containing a four-point (0-3) response category. The possible scores range from 0 to 21 for anxiety and the same for depression. Anxiety and depression should be scored separately. A score of 0 to 7 for either subscale can be regarded as being in the normal range, a score of 8 to 10 suggests a borderline case, and a score of 11 to 21 suggests an abnormal case.32–34
The validated Portuguese version of the Kansas City Cardiomyopathy Questionnaire (KCCQ), a responsive health-related QoL measure for HF, was also used.30 The KCCQ is a 23-item, self-administered instrument that quantifies two main domains composed of multiple subdomains: symptoms (physical function and symptoms frequency, severity and recent change), and global QoL (social function, self-efficacy and knowledge and quality of life). Scores are transformed to a range of 0-100, in which higher scores reflect a better health status.35,36
The KCCQ was answered at hospital discharge (referring to the QoL status previous to hospital admission), and during follow-up at three months and between the 6th and the 12th month after discharge.
Demographic and clinical data, including laboratory values, echocardiography and electrocardiography results, and other instrumental data were collected from the patient's clinical file.
Prognostic outcomes were assessed as a composite endpoint of all-cause readmissions or mortality and individual endpoints of all-cause readmissions and HF readmissions. The association of CI with QoL was also evaluated. The occurrence of death and rehospitalization and the cause of rehospitalization were identified based on the information available in the clinical records.
Statistical analysisStatistical analysis was performed using IBM® SPSS® Statistics 20 (Chicago, IL, United States). Categorical variables were reported in absolute numbers and percentage and continuous variables were reported as mean and standard deviation (SD) or median and interquartile range. The impact of cognitive status on endpoints was assessed using the statistical methods of Cox Regression and Kaplan-Meier survival analysis. Anxiety and depressive status were used as covariates and included together with cognitive status (evaluated by MoCA and HADS questionnaires) on multivariate analyses (adjusted for age, left ventricle ejection fraction (LVEF) and NYHA functional class at discharge). Wilcoxon's test was used to evaluate MoCA value variation during follow-up. P<0.05 was considered statistically significant.
Ethical considerationsThe study was approved by the Institutional Ethics Committee and by the National Committee on Data Protection. Patient confidentiality was ensured through the anonymization of the collected data and written informed consent was obtained from all patients before enrolment. All study procedures were carried out in accordance with the ethical principles of the 2013 revised version of the Declaration of Helsinki.37
ResultsBaseline dataA total of 43 patients were included in the present study. The mean follow-up period was 8.2±2.1 months. Patient demographic and clinical characteristics at baseline are described in Table 1. Mean age was 67±11.3 years and 31 patients (68.9%) were male. The most frequent HF etiology was idiopathic dilated cardiomyopathy (55.8%), followed by ischemic heart disease (23%). Comorbidities (systemic hypertension, chronic kidney disease, atrial fibrillation and diabetes) were highly prevalent.
Demographic and clinical data of the study population at baseline.
| Age - years (mean±SD) | 67±11.3 |
| Male gender- n (%) | 31 (68.9) |
| Patients with <12 years of education - n (%) | 28 (65.1) |
| HF etiology - n (%) | |
| Ischemic heart disease | 10 (23.3) |
| Valvular heart disease | 5 (11.6) |
| Idiopathic dilated cardiomyopathy | 24 (55.8) |
| Hypertrophic cardiomyopathy | 2 (4.7) |
| Restrictive/infiltrative cardiomyopathy | 1 (2.3) |
| Other | 1 (2.3) |
| NYHA – on admission - n (%) | |
| I | 0 |
| II | 2 (4.7) |
| III | 21 (48.8) |
| IV | 20 (46.5) |
| NYHA - at discharge - n (%) | |
| I | 12 (27.9) |
| II | 29 (67.4) |
| III | 2 (4.7) |
| IV | 0 (0) |
| LVEF (mean±SD) at discharge | 30.3±12.5% |
| HFrEF (n (%)) | 37(86%) |
| HFmrEF (n (%)) | 3 (7%) |
| HFpEF (n (%)) | 3 (7%) |
| Comorbidities- n (%) | |
| Diabetes | 14 (32.6) |
| Systemic hypertension | 31 (72.1) |
| Chronic kidney disease (eGFR<60ml/min) | 18 (41.9) |
| Atrial fibrillation | 24 (55.8) |
| Blood pressure at discharge (mean) | |
| Systolic blood pressure | 107 mmHg |
| Diastolic blood pressure | 59 mmHg |
| HF therapy prior to admission - n (%) | |
| ACEi/ARB | 25 (58.1) |
| Beta-blocker | 25 (58.1) |
| MRA | 10 (23.3) |
| Sacubitril-valsartan | 0 (0) |
| CRT | 9 (20.9) |
| Laboratory plasma or serum values (median) on admission | |
| NTproBNP | 4222 pg/mL |
| Serum creatinine | 1.0 mg/dL |
| Hemoglobin | 14.0 g/dL |
| HbA1c | 6.0% |
| Total bilirubin | 1.0 mg/dL |
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CRT: cardiac resynchronization therapy; eGFR: estimated glomerular filtration rate (by CKD-EPI creatinine equation); HbA1C: Hemoglobin A1C; HF: heart failure; HFrEF: HF with reduced LVEF; HFmrEF: HF with LVEF in the median range; HFpEF: HF with preserved LVEF; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N terminal proB-type natriuretic peptide; NYHA: New York Heart Association functional class.
The median LVEF assessed by transthoracic echocardiography was 30.3±12.5%, and 37 (86%) patients had HF with reduced ejection fraction (HFrEF). Prior to index-hospitalization, 25 patients (58%) were on treatment with an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), 25 (58%) with a beta-blocker and 10 (23%) with a mineralocorticoid receptor antagonist. Index-hospitalization was the first HF admission for 17 patients (39.5%). Nearly half of the patients (48.8%) were in NYHA functional class III on admission and 95% were discharged in NYHA functional class I or II. The mean length of stay was 14.6±16.9 days. Neurohormonal modulation therapy was successfully initiated and/or up-titrated during the hospital stay and optimized treatment (drugs, and devices if indicated) was attempted in all patients during follow-up. The mean score on MoCA at-discharge was 22.6±4.2 and 11 patients (25.6%) had an abnormal score affecting mainly the cognitive domains of visuospatial/executive capacity, language and delayed recall (Table 2, Figure 1).
Cognitive domains – individual scores of the study population.
| Cognitive domain | Baseline (at discharge) | Follow-up | P | ||
|---|---|---|---|---|---|
| Mean score (% of attainable total) | Number of patients with maximum score | Mean score (% of attainable total) | Number of patients with maximum score | ||
| Visuospatial/executive | 63.2±26.1 | 7 (16.3%) | 62.9±25.4 | 8 (18.6%) | 0.34 |
| Naming | 97.4±9.0 | 40 (93.0%) | 90.5±17.8 | 32 (74.4%) | 0.031 |
| Attention | 82.5±22.2 | 16 (37.2%) | 79.8±28.1 | 22 (51.2%) | 0.081 |
| Language | 70.3±27.7 | 22 (51.2%) | 75.0±23.4 | 17 (39.5%) | 0.079 |
| Abstraction | 79.0±34.2 | 14 (32.6%) | 76.8±31.9 | 26 (60.5%) | 0.78 |
| Delayed recall | 46.8±32.3 | 2 (4.7%) | 55.7±26.8 | 3 (7.0%) | 0.27 |
| Orientation | 94.7±8.8 | 31 (72.1%) | 92.3±17.3 | 34 (79.1%) | 0.18 |
A better performance on MoCA score at discharge was achieved by the male population (p=0.026) and by patients on ACEi or ARB prior to the index-hospitalization (p=0.009).
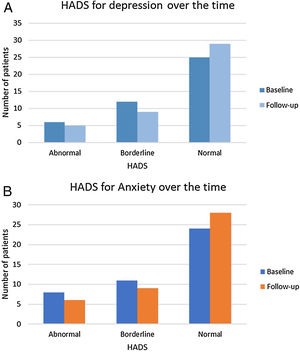
At discharge, six patients (14%) had an abnormal HADS for depression, 12 patients (27.9%) obtained a borderline value, and 25 patients (58%) had a normal score.
Regarding HADS for anxiety, 24 patients (55.8%) had a normal score at discharge, 11 patients (25.6%) had a borderline score, and 8 (19%) had an abnormal score.
Neither depression nor anxiety screened by HADS was associated with an abnormal MoCA score.
Follow-up dataFollow-up clinical data are detailed in Table 3. When comparing the results of the MoCA scores at baseline and at the follow-up evaluations, no significant difference was found (mean scores: 22.6±4.2 vs. 22.2±5.5; p=non-significant [NS]), and the total number of patients with an abnormal score was also similar (11 (25.6%) vs. 10 (23.3%); p=NS). However, a significant reduction in the naming ability domain was observed during follow-up (97.4±9.0% vs. 90.5±17.8%; p=0.031) but this result was not considered clinically relevant (Table 2).
Clinical data of the study population at follow-up.
| NYHA - n (%) | |
| I | 31 (72.09) |
| II | 10 (23.4) |
| III | 1 (2.33) |
| IV | 1 (2.33) |
| LVEF (mean±SD) | 40.8±11.7% |
| LVEF<40% (n (%)) | 19 (44.2%) |
| LVEF between 40-49% (n (%)) | 14 (32.6%) |
| LVEF>50% (n (%)) | 10 (23.3%) |
| Blood pressure (mean) | |
| Systolic blood pressure | 109 mmHg |
| Diastolic blood pressure | 61 mmHg |
| HF therapy - n (%) | |
| ACEi/ARB/sacubitril-valsartan | 43 (100) |
| Beta-blocker | 40 (93.0) |
| MRA | 35 (81.4) |
| CRT | 9(20.9) |
| Laboratory plasma or serum values (median) | |
| NTproBNP | 1620 pg/mL |
| Serum creatinine | 1.0 mg/dL |
| Hemoglobin | 14.0 g/dL |
| HbA1c | 6.0% |
| Total bilirubin | 1.0 mg/dL |
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CRT: cardiac resynchronization therapy; HbA1C: Hemoglobin A1C; HF: heart failure; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N terminal proB-type natriuretic peptide; NYHA: New York Heart Association functional class.
The mean HADS for depression and anxiety did not change significantly during follow-up (6.7±4.0 vs. 5.63±4.5; p=0.137; 6.98±4.4 vs. 6.57±4.4; p=0.624, respectively). However, some individual variation was found. During follow-up, 29 (67.4%) patients had a normal HADS for depression, 9 (20.9%) a borderline value and 5 (11.6%) an abnormal score; and 28 (65.1%) patients had a normal HADS for anxiety, 9 (20.9%) a borderline value and 6 (14.0%) an abnormal score (Figure 2).
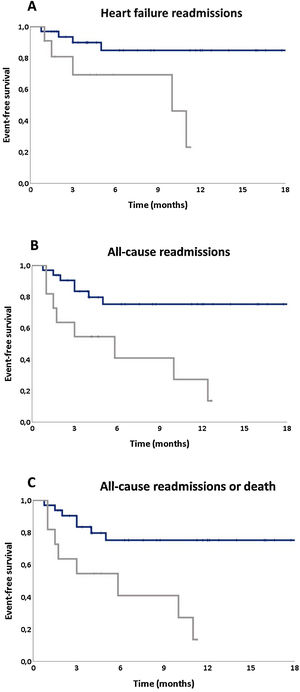
Influence of cognitive status in heart failure outcomesDuring follow-up, one patient died (2.33%), 14 (32.6%) patients where readmitted (20.9% due to HF) and 15 (34.9%) patients had the composite endpoint of all-cause rehospitalization and/or mortality. The presence of mild CI at discharge (reflected by a MoCA <22) was highly predictive of adverse events during the follow-up period: in a multivariate analysis a MoCA score <22 (vs. ≥22) at discharge increases sixfold the risk of HF readmissions (HR=6.42 [1.23-32.61]; p=0.025) (Figure 3A). Also, the presence of CI was an independent predictor of all-cause readmissions (Hazard ratio [HR]=4.00 (1.15-13.95); p=0.03) (Figure 3B) and conferred an almost five fold greater risk of the composite endpoint of all-cause readmission or death (HR=4.63 [1.37-15.67]; p=0.014) (Figure 3C).
There was no significant difference in KCCQ main domains (symptoms and global QoL) when comparing patients with vs. without CI (65.1±26.3% vs. 64.9±25.2%; p=NS; 60.5±18.4% vs. 64.4±19.4%; p=NS, respectively), but patients with a higher MoCA score performance at discharge, and therefore with better cognitive status, had a better ability to deal with their HF according to the assessment of this component in the KCCQ (p=0.038).
DiscussionTo our knowledge this is the first longitudinal study performed in a cohort of Portuguese patients with HF that assessed cognitive status simultaneously with anxiety and depression, its variation and prognostic impact after hospital discharge, when followed-up according to a dedicated and structured HF program.
Moreover, this study adds information to previous published data regarding the evolution of cognitive status, anxiety and depressive status (assessed with simple-to-apply although recommended screening tools) in a group of patients managed according to the most recent recommendations for HF treatment. According to our findings, cognitive status, anxiety and depression remained stable over almost one year of follow-up under optimized HF therapy; mild CI had significant impact on the patient's prognosis and poorer quality of life. Specific contributions are discussed in the following paragraphs.
The presence of CI in the HF population is well documented in literature,38,39 with most studies including patients with heart failure with reduced ejection fraction. Higher prevalence is observed in patients with more severe symptoms and need for hospitalization.40–43 Our population, showing a significant rate (26%) of mild CI, fits this profile, as 86% had HFrEF, all were discharged from hospital after an episode of HF decompensation, and 60% had previous hospitalizations for HF.
Although this association between CI and HF has been well documented in the literature, with HF patients presenting a twofold increased risk of impaired cognitive function compared with age-matched controls,20 the understanding of the time course of cognitive change in HF is limited.14,20 Few studies have examined longitudinal neurocognitive outcomes in HF19 and their results are contradictory,20 with some studies showing an improvement in cognitive status over time, while others document a deterioration, or that cognitive state remains stable. Our study helps to clarify this controversial topic.
First, we present a study with longitudinal design that shows a minimal improvement in cognitive performance (from 26% to 23% of patients with CI, not statistically significant) assessed with a screening tool (MoCA score) during follow-up. This result is in line with Riegel et al.’s study that included a higher number of patients (n=279) with chronic HF followed with a battery of neuropsychological tests at baseline over a similar period of time (three and 6 months), and where the average digit symbol substitution task scores improved minimally from 53.4±17.5 at baseline, to 55.8±17.6 at three months, and to 58.1±17.9 at six months.21
Others14,16,19 have found significant improvements in cognition over time. However, those patients had higher LVEF and were assessed at different stages of HF decompensation, therefore different patient profiles and settings might explain the different results. In addition, other studies that compared patient follow-up scores on cognitive tests with their baseline scores and tended to report significant improvements in cognitive performance over time, usually show significant improvements in cardiac parameters as a result of treatments or interventions tested in the study,44–47 or a different design to our study which is just observational. This association was most evident in studies documenting dramatic improvements in cardiac function, such as those assessing heart transplantation recipients.20,21,46,47
As previously mentioned, there is no consensus on this topic, as other previous studies reported decreased cognitive function over time in HF patients.23 Almeida et al. studied 77 adults with HF (defined by LVEF<40%) with a battery of neuropsychological tests (Cambridge Cognitive Examination of the Elderly (CAMCOG) and found that HF patients showed evidence of cognitive decline over two years compared with controls with normal left ventricular function; again a study with a different design and with a longer follow-up.
To sum up, we can put forward some hypotheses to explain our results: first, our follow-up of eight months might be too short to show significant changes in the cognitive status; second, although the recommended HF therapy improves the prognosis in these patients, it may not have had impact on some of the mechanisms of CI in HF, particularly on the deranged self-regulation of brain vessels and metabolism and on the systemic inflammatory and hypercoagulability states associated with HF; and finally, CI might represent an irreversible impairment in some patients, and optimal medical therapy may only contribute to stabilization, preventing its worsening.
Second, we found a significant impact of cognitive status on HF patient prognosis, even when assessed with a screening tool. The presence of CI (assessed by an abnormal MoCA score) at discharge conferred a fivefold greater risk of the composite endpoint of all-cause readmissions or death, increasing by four times the risk of hospital readmission and by six the risk of HF readmission. Also, patients with a better MoCA score performance at discharge, reflecting a better cognitive status, were better placed to deal with their heart disease. The results regarding outcomes are in line with those observed in several previous larger studies48 showing a strong independent association of CI with increased mortality and readmissions in HF patients,10,49–52 even in cases of only mild CI10,50 that often remain undiagnosed. However, despite its recognized prevalence and negative prognostic impact, HF guidelines relating to cognitive assessment1,53 are scarce and no standardized procedures for assessment and management are specifically recommended.
Finally, in what concerns depression and anxiety, our study also adds to previous studies the simultaneous evaluation (easily feasible) of cognitive function, depression and anxiety (the latter very rarely studied in this population) over time in HF patients, co-morbidities with an already known relevant impact on prognosis and quality of life in these patients.11
In our study more than 40% of the patients had altered HADS result, reflecting, as previously described, that anxiety and depression are common comorbidities in HF patients.54 However, contrary to others,54 we found that these comorbidities were not associated with cognitive status. There are however some contradictory results, with some studies presenting data similar to ours.40
In this study, both depression and anxiety remain stable during follow-up. This is a relevant information as, even though they are common in these patients, these co-morbidities, especially anxiety, are poorly studied in HF.
Limitations and strengthsThe authors recognize some limitations in their work, including the fact that this study was from a single center and included only 43 patients, which may also compromise the statistical analysis in a model with several covariates. However, this represents real-world practice including an HF population admitted to hospital due to acute HF and followed-up prospectively in an outpatient HF clinic setting. The observed data on rates of cognitive impairment, anxiety and depression were similar to those obtained in studies with larger HF populations. It is plausible that the 8±2 month period of follow-up may not have been long enough to capture changes in cognitive performance in HF even with therapeutic optimization, however, some of the previously published studies have similar or shorter follow-up.20 Similar studies with a longer follow-up are needed. Another concern is the possibility of false positives and negatives in MoCA and HADS results. Also, although patients with previously diagnosed neurological or psychiatric disease were excluded, brain imaging studies were not routinely performed, so the presence of chronic brain lesions that might contribute to our results cannot be fully ruled out. Nevertheless, no patients had evidence of neurologic deficits, either at inclusion or during the follow-up period.
It is also important to note that most of the previous published studies on the prognostic impact of CI in HF used batteries of neuropsychological tests instead of screening tools.7 Although neuropsychological tests provide information about function across multiple domains and have diagnostic acuity, their application usually takes several hours and requires specialized training in the instruments and interpretating them.55 Cannon et al.7 previously recommended an increasing use of cognitive assessment with standardized screening tools in all future HF studies. These cognitive screening tools provide initial insight into an individual's cognitive status and assist with a decision for referral for more comprehensive neuropsychological testing where the presence of CI will be determined.
ConclusionThe course of cognitive change in HF is not completely understood. According to our study and in keeping with previously published works, at the moment it does not appear that the cognitive performance of HF patients greatly improves over time. Our study contributes to support the findings of previous studies on a controversial subject, indicating further areas for future investigation on this issue.
This study helps to draw attention to the significant rates of abnormal cognitive status in Portuguese HF patients which correlates with poor long-term prognosis, even in patients who are managed in accordance with best practices. These results also emphasize the need for the early identification of these conditions and underline the importance of making health professionals aware of this reality, which may lead to a more individualized approach with potential prognostic benefits.
Conflicts of interestThe authors have no conflicts of interest to declare.