The number and complexity of percutaneous interventions for the treatment of structural heart disease has increased in clinical practice in parallel with the development of new imaging technologies, in order to render these interventions safer and more accurate. Complementary imaging modalities are commonly used, but they require additional mental reconstruction and effort by the interventional team.
The concept of fusion imaging, where two different modalities are fused in real time and on a single monitor, aims to solve these limitations. This is an important tool to guide percutaneous interventions, enabling a good visualization of catheters, guidewires and devices employed, with enhanced spatial resolution and anatomical definition. It also allows the marking of anatomical reference points of interest for the procedure.
Some studies show decreased procedural time and total radiation dose with fusion imaging; however, there is a need to obtain data with more robust scientific methodology to assess the impact of this technology in clinical practice.
The aim of this review is to describe the concept and basic principles of fusion imaging, its main clinical applications and some considerations about the promising future of this imaging technology.
O número e a complexidade das intervenções percutâneas no tratamento de doenças cardíacas estruturais têm vindo a aumentar na prática clínica, estando associado ao desenvolvimento de novas tecnologias de imagem para intervenções mais precisas e seguras. Modalidades de imagem complementares são comumente utilizadas, contudo exigem um esforço de reconstrução mental por parte da equipa de intervenção.
O conceito de imagem de fusão, onde duas modalidades de imagem são fundidas em tempo real e num só monitor, vem colmatar essas limitações. Esta é uma importante ferramenta para a monitoração das intervenções percutâneas pela associação da correta visualização dos cateteres, fios-guia e dispositivos utilizados a uma melhor resolução espacial e definição anatómica. Permite também a marcação de pontos de referência com interesse anatómico para o procedimento.
Alguns estudos revelam a diminuição da duração de procedimento e da dose total de radiação; contudo, persiste a necessidade de obtenção de dados com metodologia científica mais robusta para aferir o impacto desta tecnologia na prática clínica.
Esta revisão pretende abordar o conceito e os princípios básicos da imagem de fusão, as suas principais aplicações clínicas, bem como algumas considerações acerca do futuro promissor desta tecnologia de imagem.
Interventional Cardiology is a rapidly developing field, and as advances in interventional techniques emerge, so too does the need for improved procedural efficacy and safety.1,2 Fluoroscopy is the primary imaging method used, offering good visualization of the devices employed, but it is limited in terms of anatomical assessment.
The basic concept of fusion imaging is to overlay, on the same screen, one imaging modality with good soft tissue definition, for example two-dimensional (2D) or three-dimensional (3D) transesophageal echocardiography (TEE), intracardiac echocardiography (ICE), computed tomography (CT), cardiac magnetic resonance imaging (MRI), among others, onto the image provided by the fluoroscopy, in order to compensate for its limited anatomical accuracy.3,4
This literature review addresses the concept and basic principles of fusion imaging, as well as its primary applications and the clinical results described in the literature.
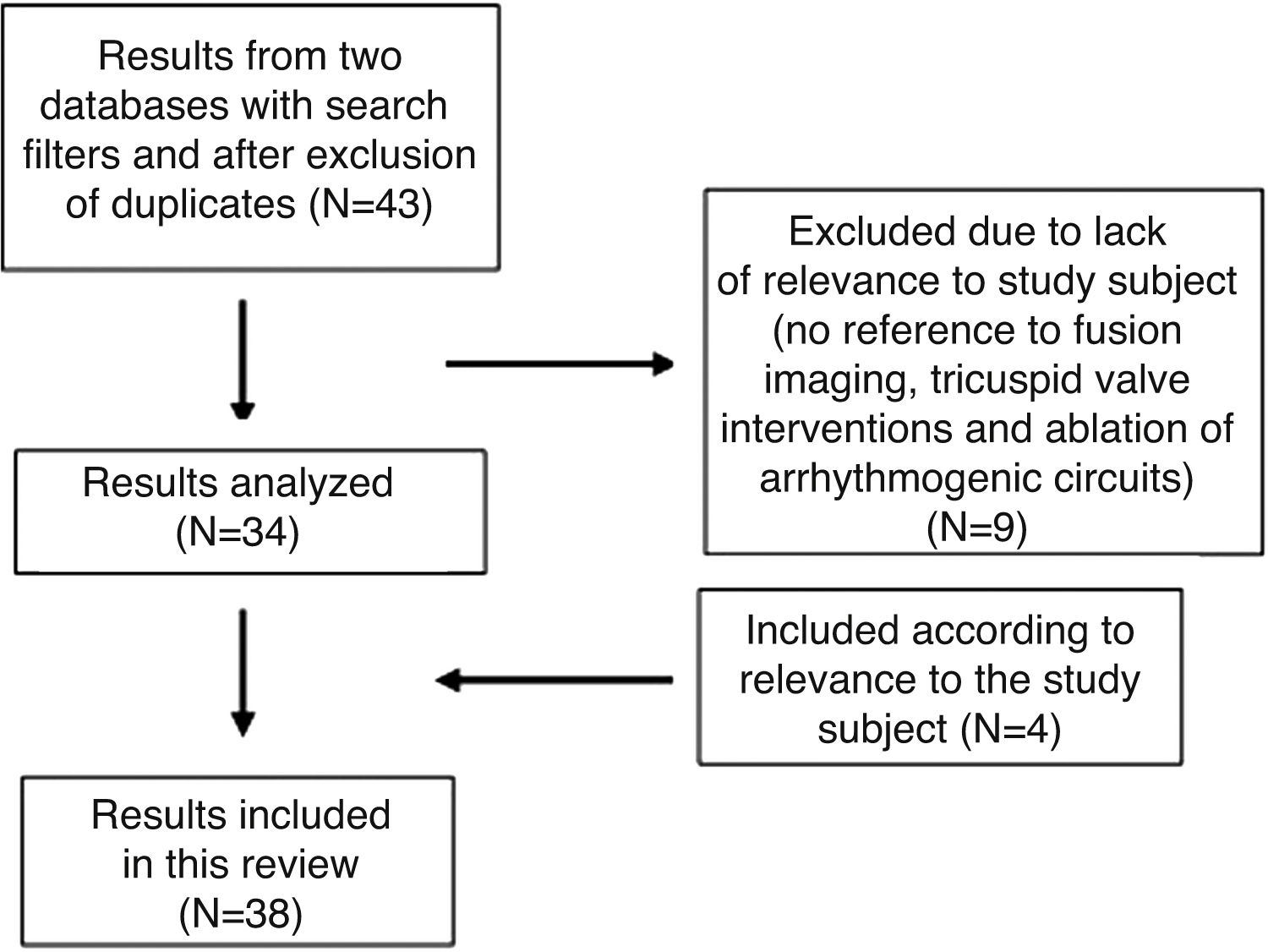
MethodologyThe bibliographical research was carried out on PubMed and Embase. The search filters used were date (published in the last five years), language (publications in English, Portuguese, French, Spanish and Italian) and species (human). On Embase, in addition to the above criteria, the bibliography was also filtered by study type, with the selection restricted to original articles, reviews and ahead of print articles.
The research was carried out in two ways: by linking the research topic (“fusion imaging” OR “hybrid imaging” OR “multimodality imaging”) to the key words fluoroscopy, transesophageal echocardiography, computed tomography, magnetic resonance and also via the keyword EchoNavigator® (Figure 1). A medical subject headings (MeSH) search was not carried out on PubMed as the fusion imaging concept does not have an MeSH term assigned.
Fusion imagingStaticThe fusion of different static imaging modalities is not a new concept: myocardial perfusion scintigraphy with CT and positron emission tomography with CT1 are some examples of static fusion imaging already established in clinical practice.1,5,6 The static nature of these modalities, however, makes their use in interventional procedures in real time somewhat limited.1,2,6
Static imaging modalities can be integrated/fused with dynamic fluoroscopy imaging, with a view to improving navigation during the procedure through better anatomical mapping.2,5 This requires images obtained in advance to be taken in the same position in which the patient will undergo the percutaneous intervention.6 The imaging most commonly combined with fluoroscopy is CT, and it is most frequently used in transcatheter aortic valve replacement (TAVR), percutaneous paravalvular leak closure and percutaneous left atrial appendage occlusion (LAAO) procedures.2 Rotational angiography with 3D reconstruction (RA-3D) is another modality that can be obtained in advance and subsequently fused with the fluoroscopy. This entails acquiring the image, segmentation into a 3D model, marking points of anatomical interest/virtual mapping, and then co-registering the 3D model with the fluoroscopy to overlay the image. As such, the previously obtained 3D model and the points of interest marked will move in sync with the C-arm, improving anatomical and spatial resolution during procedures.4,6
Glöckler et al. analyzed 78 percutaneous interventions with 3D imaging navigation (RA-3D, MRI, CT), 12 of which were angioplasties with stenting in aortic coarctation. Using a prospective observational methodology, they compared the fluoroscopy time, radiation dose and contrast volume with 20 control cases from a historical cohort study (fluoroscopy). They did not find significant differences in the radiation or contrast doses, but a reduction in fluoroscopy time was indicated (8.33 vs. 10.2 min; p=0.04).7 This fusion imaging modality can be used in coronary interventions, pulmonary artery angioplasty, pulmonary valve interventions and also in TAVR.1,2
Another example of fusion imaging is MRI-fluoroscopy, the main advantage of which is the absence of ionizing radiation involved in obtaining MRI imaging.2,8 The ability of MRI to incorporate cardiac and respiratory movement is another benefit which improves procedural efficacy by allowing better alignment between the two images.9 Clinical applications include endomyocardial biopsies (right ventricular wall) and electrophysiology procedures.4,8
DynamicTEE is a dynamic imaging modality that offers good visualization of anatomical structures on multiple planes and with good definition, without the need for contrast and/or ionizing radiation,4 as well as functional assessment by Doppler.10 However, it has limited spatial resolution, is operator-dependent, requires sedation and has very limited capacity to detect the devices used during the intervention. All these limitations can be solved by fluoroscopy.2,4,3
These two images are routinely obtained independently and displayed on different screens in different orientations, which requires the information to be integrated and mentally reconstructed by the operators.3,11 In 2014, the second version of the EchoNavigator® software (Philips Healthcare, Best, The Netherlands) was developed, which enables TEE to be fused with the fluoroscopy imaging on the same screen.2,12 Once the TEE ultrasound has been automatically co-registered, repositioning the C-arm allows the fluoroscopic and echocardiographic images to move in sync, without loss of imaging fusion.1,4,10,3
The co-registration process uses a calibration algorithm between the TEE ultrasound and the fluoroscopy for the system to identify the ultrasound probe and generate an overlay image with the two modalities orientated in the same direction.5 This is automatic and fairly accurate (the average error is one to two milimeters),11 and can be optimized by placing the TEE probe in the center of the fluoroscopy image screen, at the following angles: 0°, 45° left anterior oblique and 45° right anterior oblique.11 Once registration is complete, all changes to the TEE probe (position, rotation and angulation) are automatically detected by the system and the image is updated on the screen, together with the fluoroscopy. Changes to the position of the C-arm also alter the position of the TEE images.2,10,11
Marking virtual points of anatomical interest that are relevant to the intervention is one advantage of this software.10,11 Once the probe is co-registered, various points can be marked on the echocardiographic image which are automatically synchronized with the fluoroscopy, where they remain fixed independent of any change to the position of the ultrasound.5,10,11 However, it is important to be aware of movements that are vertical to the table and the position of the patient, as they can cause points already marked to be lost or even require the TEE probe to be co-registered again.5,11 As the TEE probe, after the initial registration, can be left for long periods without being used, this can also cause these virtual markers to be lost.5,10 Its static nature is ultimately a limitation, as it does not respond to the translational movements of the patient (respiration, positioning) nor to tissue deformation.5,10
The EchoNavigator® software allows visualization in different imaging modes: echo view, C-arm view, X-ray view and free view. In echo view the TEE image is displayed, and this is exclusively under the control of the TEE probe operator.4,5,10,11 In the C-arm view the TEE image is also displayed, but with C-arm navigation. In X-ray view we see the full fusion imaging of the TEE with the classic fluoroscopy, with or without the markings previously made. Note that all changes to the position of the TEE probe are automatically registered and updated on the fluoroscopy.5 Finally, in free view we see the TEE imaging with no specific orientation, which allows the images to be directly rotated, modified and repositioned during the intervention onto the plane which results most favorable technically.4,10,3,11,13
Clinical applications in percutaneous interventionsTransseptal punctureTransseptal puncture (TSP) is an essential first step in many percutaneous interventions. It must be carried out in the fossa ovalis and, depending on which intervention will follow, the puncture can be made in different positions.1,5,14
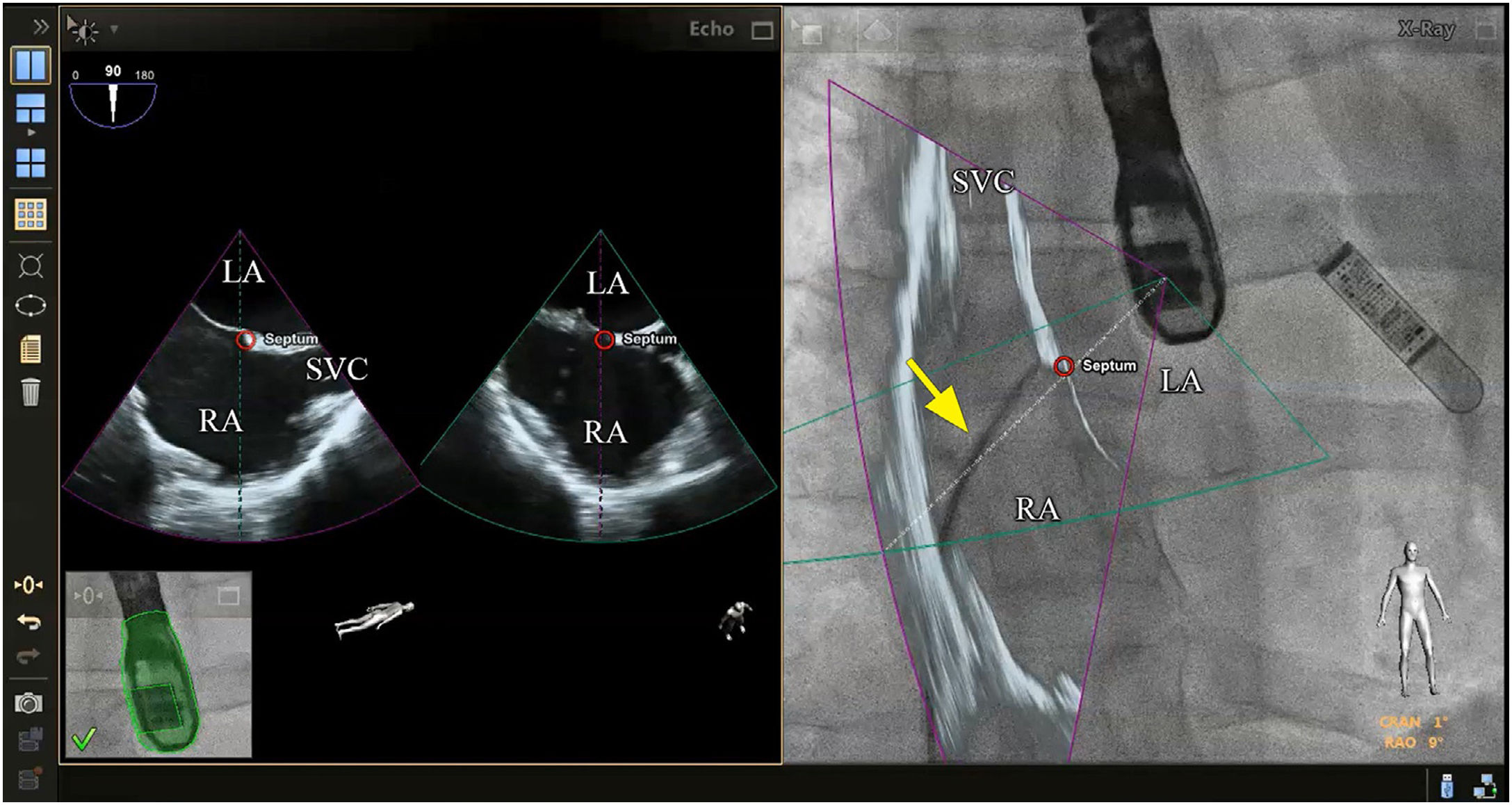
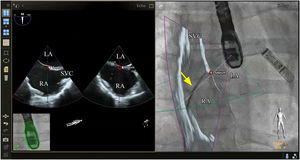
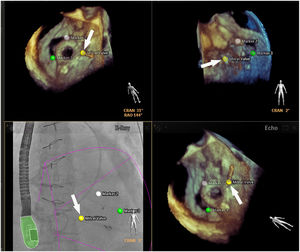
Although there is no scientific evidence as yet that supports the use of fusion imaging in this context, the technique is promising14 in terms of improved anatomical visualization in real time of the fossa ovalis, the ability to mark virtual anatomical points and, consequently, of the greater security it gives cardiologists (Figure 2).10,3 Faletra et al. reviewed the main advantages of fusion imaging in their clinical experience, notably the accuracy of the puncture site, the projection of the fluoroscopy image adapted to the patient and the optimization of hand-eye coordination. It was not clear if it reduced the total duration of the intervention or the incidence of complications compared with traditional TEE.14 As a result, they suggested the need for further studies to investigate the added clinical value of this new technology.
Fusion imaging for transseptal puncture. Left: biplane TEE. Right: fusion imaging of TEE onto fluoroscopy with transseptal catheter (yellow arrow). There is a marker in both images (red circle), placed in TEE (right) and automatically transferred to fusion imaging (left). TEE: transesophageal echocardiography; LA: left atrium; RA: right atrium; SVC: superior vena cava. Image from Wiley et al.2, reproduction allowed.
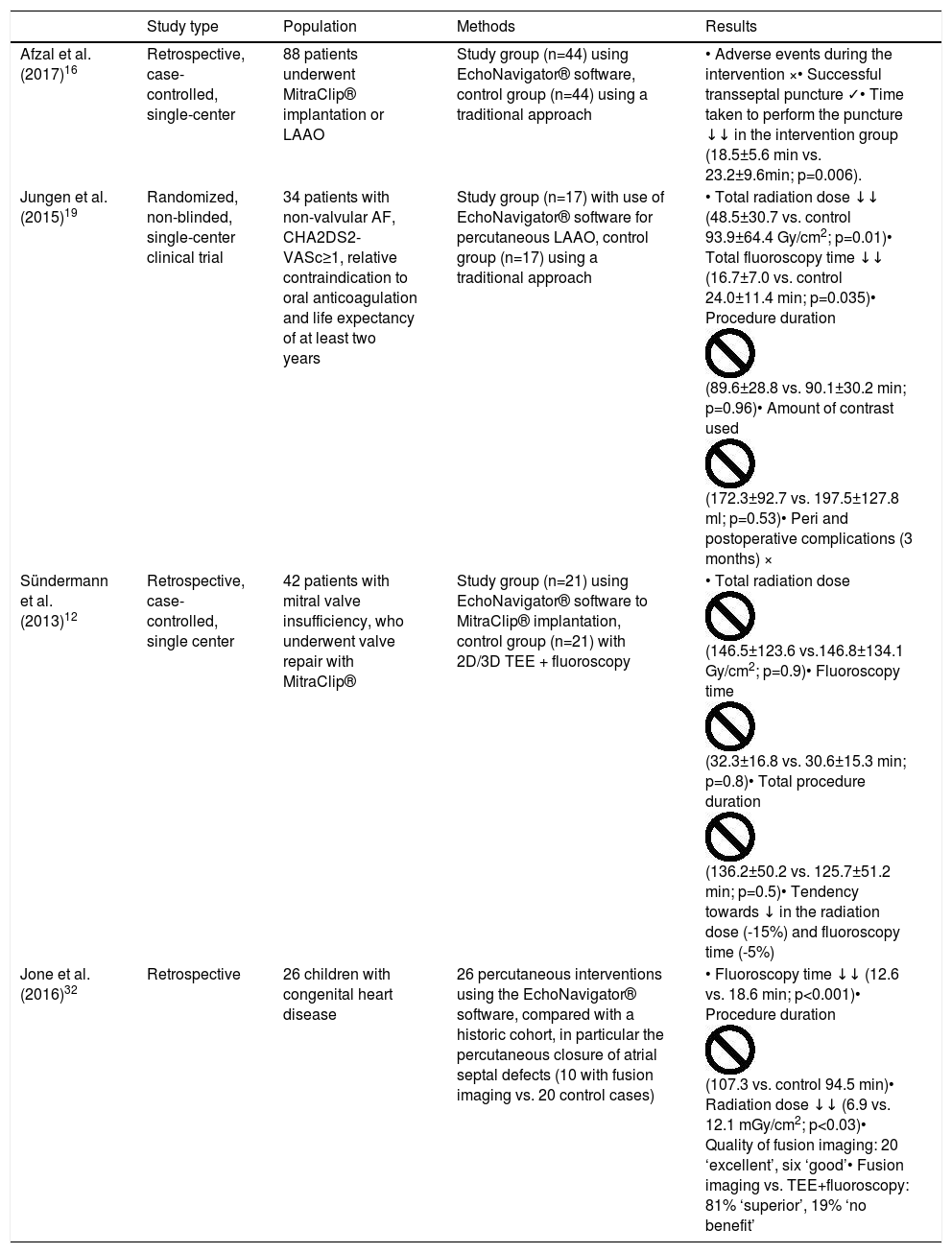
The usefulness of EchoNavigator® software has been described by some authors based on their clinical experience,14–16 but only one study specifically assessed the benefits it brought to clinical practice. Afzal et al. retrospectively assessed the use of this software during TSP in MitraClip® (Abbott, Illinois) implantation and LAAO, comparing the procedures with and without fusion.16 This study demonstrated that, with the use of fusion imaging software, there is a reduction in time (approximately 5 min, p=0.006) from the start of the procedure to the completion of the puncture, but did not, however, show differences in the incidence of adverse events nor in procedure success (see Table 1).11,16
Summary of the main studies.
| Study type | Population | Methods | Results | |
|---|---|---|---|---|
| Afzal et al. (2017)16 | Retrospective, case-controlled, single-center | 88 patients underwent MitraClip® implantation or LAAO | Study group (n=44) using EchoNavigator® software, control group (n=44) using a traditional approach | • Adverse events during the intervention ו Successful transseptal puncture ✓• Time taken to perform the puncture ↓↓ in the intervention group (18.5±5.6 min vs. 23.2±9.6min; p=0.006). |
| Jungen et al. (2015)19 | Randomized, non-blinded, single-center clinical trial | 34 patients with non-valvular AF, CHA2DS2-VASc≥1, relative contraindication to oral anticoagulation and life expectancy of at least two years | Study group (n=17) with use of EchoNavigator® software for percutaneous LAAO, control group (n=17) using a traditional approach | • Total radiation dose ↓↓ (48.5±30.7 vs. control 93.9±64.4 Gy/cm2; p=0.01)• Total fluoroscopy time ↓↓ (16.7±7.0 vs. control 24.0±11.4 min; p=0.035)• Procedure duration (89.6±28.8 vs. 90.1±30.2 min; p=0.96)• Amount of contrast used (172.3±92.7 vs. 197.5±127.8 ml; p=0.53)• Peri and postoperative complications (3 months) × |
| Sündermann et al. (2013)12 | Retrospective, case-controlled, single center | 42 patients with mitral valve insufficiency, who underwent valve repair with MitraClip® | Study group (n=21) using EchoNavigator® software to MitraClip® implantation, control group (n=21) with 2D/3D TEE + fluoroscopy | • Total radiation dose (146.5±123.6 vs.146.8±134.1 Gy/cm2; p=0.9)• Fluoroscopy time (32.3±16.8 vs. 30.6±15.3 min; p=0.8)• Total procedure duration (136.2±50.2 vs. 125.7±51.2 min; p=0.5)• Tendency towards ↓ in the radiation dose (-15%) and fluoroscopy time (-5%) |
| Jone et al. (2016)32 | Retrospective | 26 children with congenital heart disease | 26 percutaneous interventions using the EchoNavigator® software, compared with a historic cohort, in particular the percutaneous closure of atrial septal defects (10 with fusion imaging vs. 20 control cases) | • Fluoroscopy time ↓↓ (12.6 vs. 18.6 min; p<0.001)• Procedure duration (107.3 vs. control 94.5 min)• Radiation dose ↓↓ (6.9 vs. 12.1 mGy/cm2; p<0.03)• Quality of fusion imaging: 20 ‘excellent’, six ‘good’• Fusion imaging vs. TEE+fluoroscopy: 81% ‘superior’, 19% ‘no benefit’ |
Other fusion imaging modalities are also referenced in the literature,4 such as fusion between CT or RA-3D and fluoroscopy. This perfects anatomical recognition of the sites that are relevant for the intervention, but the static character of these modalities continues to be a challenge, principally due to compensation for cardiac or respiratory movements, which can lead to a considerable margin for error.4,14
Some experts believe, based on their clinical experience, that simple 2D TEE imaging during TSP is, in the majority of cases, sufficient, and fusion imaging should be reserved for more anatomically complex situations.11
Left atrial appendage occlusionLAAO was initially carried out with fluoroscopy alone, but the gold standard now includes TEE and fluoroscopy.2,3,16 ICE is an alternative to TEE which avoids the need for sedation and the complications associated with esophageal intubation.17,18 However, all these imaging modalities are displayed without direct fusion with fluoroscopy.
The literature also describes the fusion of CT or RA-3D imaging obtained in advance with fluoroscopy.19 CT offers superior spatial resolution to TEE and ICE, which provides better anatomical characterization of the LAA, of its form and dimensions, which is essential for choosing the correct type and size of device required.20 However, they are not real time images.
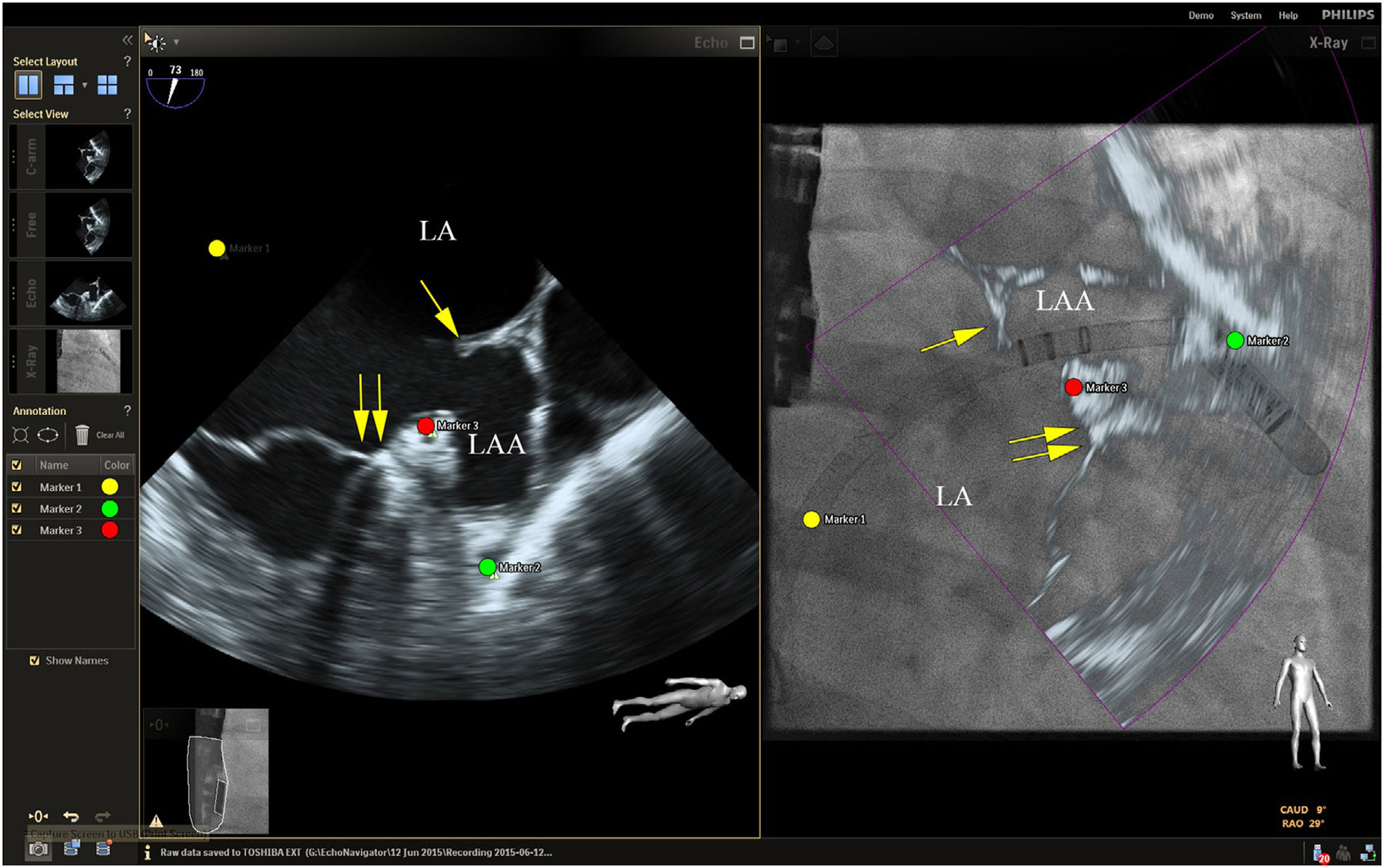
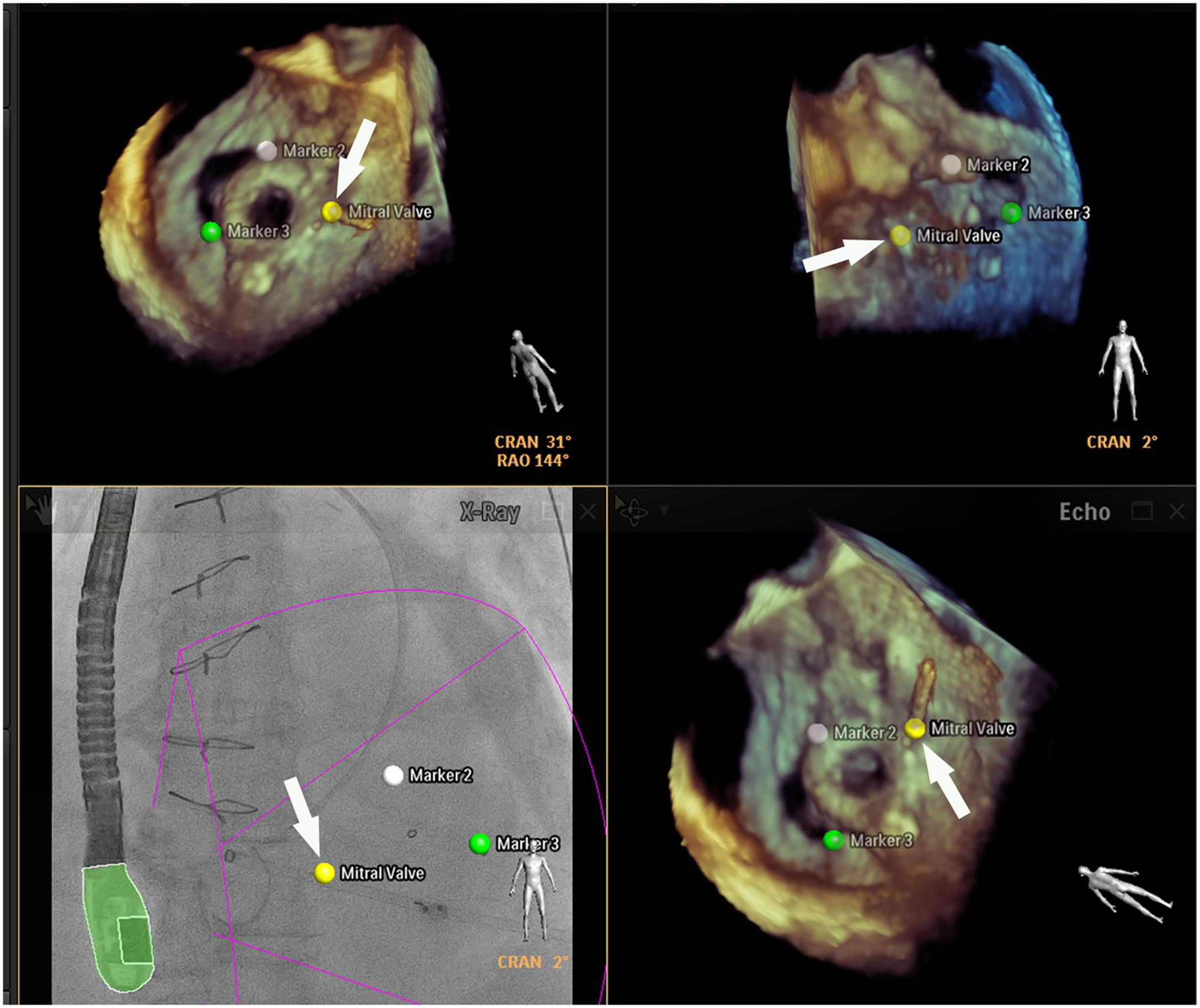
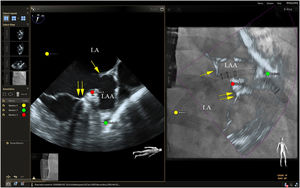
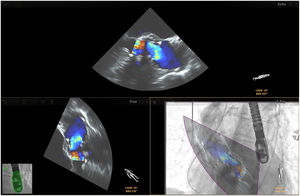
Placing reference point markers, particularly on the circumflex artery, on the orifice of the left lower pulmonary vein and on the tip of the LAA (Figure 3), allows this procedure to be carried out more safely, and there is greater advantage to be gained by placing them on TEE plus fluoroscopy imaging as they are in real time.1,2,4,5,3
Fusion imaging for LAA occlusion. Left: TEE with coumadin ridge (yellow arrow) and the mitral annulus (double yellow arrows). Right: fusion imaging of TEE and fluoroscopy, with the guide catheter passing through the optimal site for transseptal puncture (yellow) and other anatomical structures previously marked – the left circumflex coronary artery (red) and the tip of the LAA (green). TEE: transesophageal echocardiography; LA: left atrium; LAA, left atrial appendage. Image from Wiley et al.2, reproduction allowed.
Jungen et al. randomized two patient groups for this procedure, one using EchoNavigator® software (n=17) and the other using TEE (n=17).19 The total radiation dose, the fluoroscopy time, the procedure duration and amount of contrast used were measured. The incidence of peri and postoperative complications was also assessed (with a three-month follow-up). It was shown that, in the group using EchoNavigator®, the radiation dose was reduced by a half (48.5±30.7 vs 93.9±64.4 Gy/cm2; p=0.01), and the total fluoroscopy time was reduced by more than 20% (16.7±7 vs. 24.0±11.4 min; p=0.04). The procedure duration (89.6±28.8 vs. 90.1±30.2 min; p=0.96) and the amount of contrast used (172.3±92.7 vs.197.5±127.8 ml; p=0.53) did not differ significantly between the two groups. There were no intervention-related complications (Table 1).19
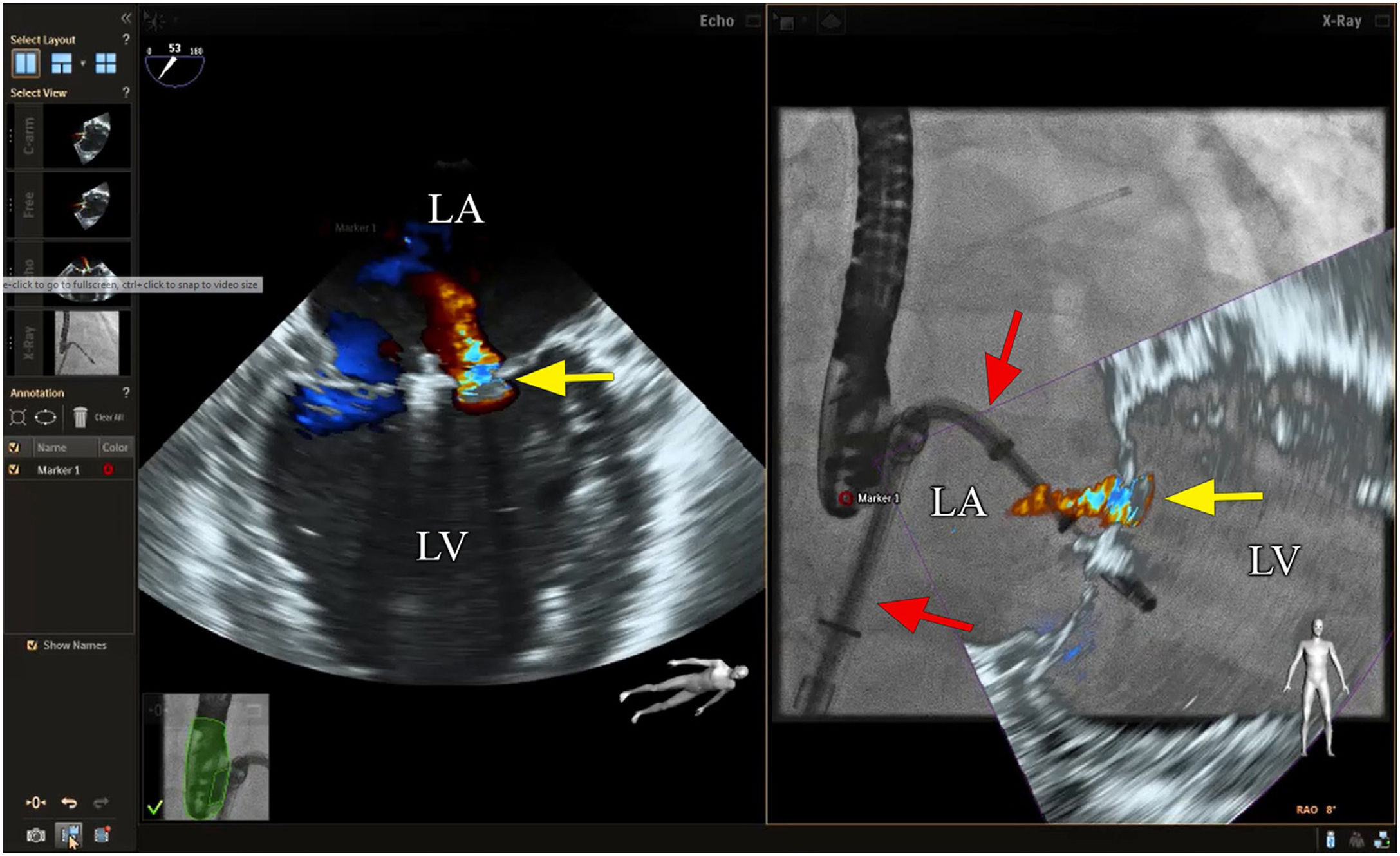
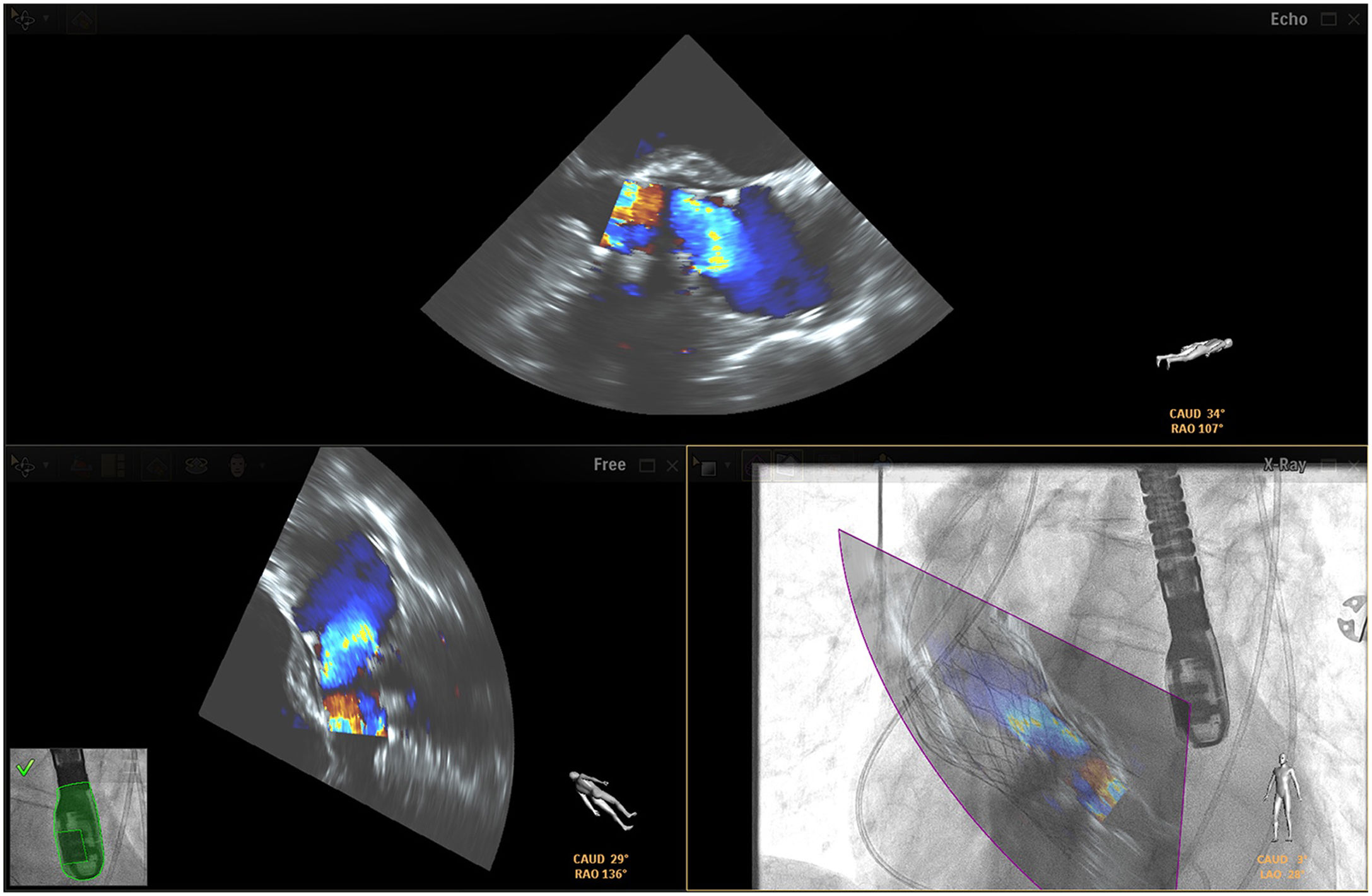
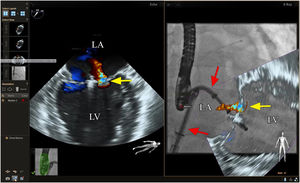
Mitral valve repairChoosing the best technique for navigating this intervention continues to be a challenge.2 The initial assessment uses TEE to provide a detailed anatomical description of the mitral valve and its adjacent structures.21 The literature has come to refer to fusion imaging as a promising solution for monitoring TSP and/or transapical puncture for access to the mitral valve and to improve the trajectory of the MitraClip® device, both for entering the atrium and for correct navigation and placement (Figure 4).5 Fusion imaging is also referred to as a way of improving the efficacy and safety of this technique,12 as it can prevent the incidence of some complications, such as injury to the aortic root and perforation of the left atrial wall.3 Postoperatively, it also plays an important role in assessing valvular competence, transmitral gradient and the position and stability of the clip.15,21
Fusion imaging for transcatheter mitral valve repair (MitraClip®). Left: TEE with color Doppler shows the mitral regurgitant jet (yellow arrow). Right: fusion imaging of TEE color Doppler onto fluoroscopy shows the guide catheter and its position relative to the regurgitant jet, facilitating precise adjustment of clip (MitraClip®) position. LA: left atrium; LV: left ventricle. Image from Wiley et al.2, reproduction allowed.
Sündermann et al. compared, via a prospective observational study, two patient groups that underwent percutaneous mitral valve repair (MitraClip®), with (n=21) and without (n=21) the use of fusion imaging with EchoNavigator® software.12 It demonstrated that the radiation dose (146.5±123.6 vs.146.8±134.1 Gy/cm2; p=0.9), fluoroscopy time (32.3±16.8 vs. 30.6±15.3 min; p=0.8) and procedure duration (136.2±50.2 vs. 125.7±51.2 min; p=0.5) did not differ significantly between the two groups.12 The authors explained that the results were not as expected due to constant software updates during the study and also due to interventions in the study group being more complex and requiring the application of more clips (45 vs. 36). Longer duration could have been expected, due to the greater complexity, but this was not observed. This suggested, therefore, that there was a trend toward a reduction in the radiation dose (-15%) and fluoroscopy time (-5%), showing it to be a viable and safe technique for this procedure (see Table 1).12
According to the clinical experience reported by some authors, another advantage of fusion imaging in this intervention is the ability to mark anatomical reference points: the puncture site on the interatrial septum (anterosuperior), crista terminalis (between the pulmonary vein and the LAA) and the center of the mitral valve.5,3,13 Also, the ability to determine, more accurately, the length of catheters and their relationship to the surrounding anatomical structures may reduce the incidence of complications during the procedure.2,11,13
Paravalvular leak correctionParavalvular leaks are one of the main complications following prosthetic valve replacement. To correct them, it is essential to assess the quantity, location, severity and form, and this is generally done using TEE in advance of the procedure.11,22–24 The access point to the leak is chosen based on its location, the anatomical characteristics of the patient and the experience of the interventional cardiologist.22 Access can be made antegrade (venous, transeptal), retrograde (arterial, transfemoral) and transapical.22,24
There is not yet consensus on the best imaging technique for navigating in real time for this procedure. Hascoet et al. reported that 3D TEE is the imaging exam used most, mainly because it offers good spatial resolution to identify and subsequently close the paravalvular leak.23 CT is also of particular interest, chiefly for preoperative planning, in order to accurately locate the valvular defect and determine its characteristics (size, form, calcification and assessment of the adjacent structures).2,4,20 It also has the advantage of displaying good spatial resolution and can later be fused with fluoroscopy during the intervention.23,24
Fusion imaging can be advantageous, especially when dealing with a number of small leaks, as they can be difficult to visualize with TEE imaging alone.4,5,10 According to the experience of some authors, fusion imaging simplifies leak closure, by making access to the valve more accurate,1 enabling access to the paravalvular defect,11,13 and assisting in locating the injury and assessing the surrounding structures. Use of color Doppler imaging can also be interesting for locating the leak in real time (Figure 5).4,3
Fusion imaging for paravalvular leaks closure. There are two paravalvular leaks, a large between the green and pink markers and a small one with a yellow landmark (white arrow). The landmarks registered in the TEE images are automatically synchronized with fluoroscopy (bottom left corner). The catheter is visible through the smaller leak (white arrow, bottom right corner). TEE: transesophageal echocardiography. Image from Basman et al.4, reproduction allowed.
However, there are no studies that support the use of fusion imaging in this intervention, and there are few clinical case reports. Some authors argue that fusion imaging may not be necessary in all interventions, and should be reserved for the most complex (e.g. leaks that are difficult to identify).2 They even advance that, at times, fusion imaging can be superfluous and distracting during the intervention, particularly if all the structures can be satisfactorily visualized with just one imaging modality.2
With respect to the marking of anatomical reference points, there is also some disparity in the literature; while some authors argue that it is viable for more accurate referencing of the paravalvular leak,22,23 others argue that it is of little consequence in this intervention, given its static character which does not take into account translational valvular movements, especially with leaking aortic valves.2,5,10
Transcatheter aortic valve replacementThe preoperative examination should include an angio-CT for improved anatomical assessment of the valvular apparatus, especially the measurement of the aortic ring, assessment of the aortic root anatomy and surrounding structures, level of calcification of the aortic valve, depth of the valve implantation and quality of access.5,3,25–28
The procedure is carried out with the assistance of fluoroscopy as the gold standard imaging modality. However, with the development of fusion imaging, RA-3D or TEE have come to be used alongside fluoroscopy in real time to aid valve implantation.29 TEE is also key to the post valve implantation stage, as it can immediately indicate if the intervention has been a success, and can assess postoperative complications, such as valve regurgitation, dissection and/or rupture of the aorta, pericardial effusion, ventricular perforation and hemorrhages, among others.4,25–27
Fusion imaging using the EchoNavigator® system has been shown to be quite viable in clinical practice, as it enables good anatomical analysis in real time, taking into account aortic valve movements4,13 and allowing the relationships between the catheters, guidewires, the prosthesis itself and the anatomical structures to be observed.2,27 The placement of markers on sites of anatomical interest (e.g. at the hinge points of the three valve leaflets13), the ability to outline the aortic ring4,3 and the correct orientation of the prosthesis are also essential elements of a successful intervention (Figure 6).2 However, some authors consider the use of fusion imaging in TAVR to be somewhat limited, primarily because this technique is intended to be increasingly minimalist with minimal sedation.4
In clinical practice, fusion imaging has been used primarily with CT obtained in advance with fluoroscopy,20,28,30 as this provides good anatomical definition for a successful intervention.4 Marked anatomical references can be subsequently overlaid with fluoroscopy; however, its static character must be taken into account and it must be remembered that it does not compensate sufficiently for movement, making it more susceptible to errors.1,4,5
Madershahian et al. studied the applicability and viability of a new software prototype, Vascular Outlining (Philips Healthcare, Best, The Netherlands), for visualization of the aortic root during TAVR, as well as for assessing the positioning of the prosthesis.31 This software provides fusion imaging between angiography and fluoroscopy during the TAVR, with the preoperative planning (anatomical assessment, measurements, etc) having been done with CT. Fifteen TAVRs were completed and assessed and all of the valve prostheses were implanted successfully. Paravalvular leaks were identified in just four patients. In all the procedures a single injection of contrast was given in the aortic root. The authors concluded that the software appears to be viable and aids accuracy in aortic valve implantation, as well as reducing the contrast volume.31
In addition, a German group reported on its experience of using a static fusion imaging system in all stages of the TAVR with the EP Navigator software (Release 5.1.1.4, Philips Healthcare, Best, The Netherlands). As well as co-registering the valvular ring and the position of the cerebral embolic protection system, the authors integrated the 3D model of the femoral access onto the fluoroscopy imaging, using a small injection of contrast to correct any errors in positioning and co-registration. The femoral puncture and wire introduction were guided by the fusion registration, thus avoiding further injections of contrast during the puncture. In this proof of concept study (n=60), the group of procedures with co-registering protocol took less time, with smaller amounts of contrast and radiation dose.32
Congenital heart diseaseIn recent years, percutaneous interventions in patients with congenital heart disease have increased.33,34 To continuously improve the safety of these interventions, new imaging methodologies have been introduced with a view to refining anatomical assessment and reducing the amount of radiation used. This aspect becomes more relevant when we consider it applies to an essentially pediatric population, which is potentially at greater risk of harm from an accumulation of ionizing radiation.35
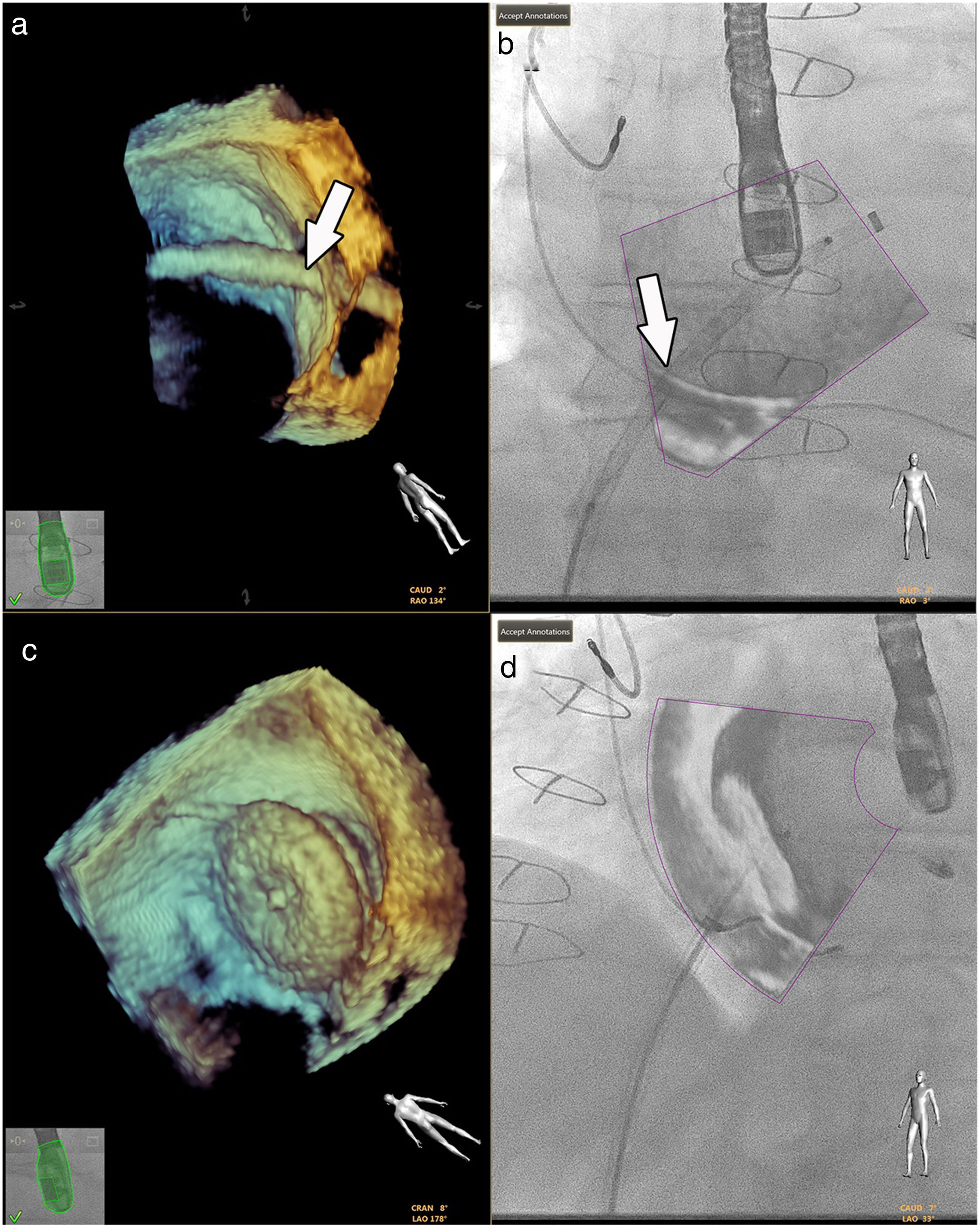
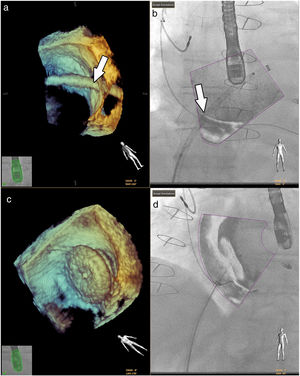
Fluoroscopy continues to be the gold standard in all interventions.33 However, good anatomical knowledge of the congenital defects is mandatory, given the small sizes often involved.1,36 Hadeed et al. assessed the viability of EchoNavigator® software in some interventions they carried out, such as percutaneous closures of atrial and ventricular septum defects, and concluded that fusion imaging between TEE and fluoroscopy had been a success in all cases, with no complications recorded (Figure 7).35 This study does not, however, supply details on the benefits in terms of procedure time or radiation doses.
Fusion imaging for guidance of atrial septal defect closure. 3D TEE image (a) of the catheter crossing the atrial septal defect (white arrow) and overlaid image with fluoroscopy in (b). 3D TEE (c) and fusion imaging (d) shows the occluder device after deployment. TEE: transesophageal echocardiography. Image from Basman et al.4, reproduction allowed.
Jone et al. sought to demonstrate the safety and efficacy of TEE plus fluoroscopy fusion imaging for navigating atrial septal defect closures in children, compared with a control group (historical cohort).34 The intervention team classified the quality of fusion imaging as excellent, good, or bad, and also graded it as superior, with no added benefit or inferior to TEE with fluoroscopy without fusion. Twenty-six interventions were carried out, 10 of which were closures of atrial septal defects involving the use of TEE plus fluoroscopy. There was a significant reduction in the fluoroscopy time (12.6 vs. 18.6 min; p<0.001) and in the dose of radiation used (6.9 vs. 12.1 mGy/cm2; p=0.03) in the group using fusion imaging. There were no statistically significant differences in the total duration of the procedure (107.3 vs. 94.5 min). The anatomical definition of fusion imaging was rated as ‘excellent’ in 20 out of 26 procedures and ‘good’ in the remaining six. Eighty percent of the procedures using fusion imaging were rated as being superior to conventional imaging, the rest being of no added benefit (Table 1).34 Although these data are encouraging, they are retrospective and based on a comparison with a historic cohort, with various sources of bias, and therefore randomized trials are needed to confirm the usefulness of fusion imaging in these interventions.
DiscussionA growing number of publications has emerged on the possible uses of fusion imaging in interventional cardiology. Dynamic fusion imaging methodologies appear to be an excellent option for improving the efficacy and safety of some procedures, resulting in a reduction in the intervention duration, fluoroscopy duration, radiation dose and volume of contrast. These data apply above all to procedures in which spatial navigation is key, such as TSP (faster with fusion imaging), LAAO (faster and with a lower radiation dose) and mitral valve repair (lower radiation dose and volume of contrast).12,16,19 Fusion imaging also appears to contribute to raising the interventional team's confidence level with regard to its own performance.13,37
The authors identify two settings in which this technology could have more potential: 1) to guide more complex cases and 2) for training teams and fellows, in which the superior anatomical and spatial characterization could be repurposed as a didactic tool for improving the learning curve. Regarding the first setting, the use of fusion imaging can increase the efficacy of very complex procedures, with a reduction in the contrast volume and radiation dose, especially in mitral valve repair techniques, paravalvular leak closures, LAAO with challenging anatomy and congenital heart disease. In the emerging field of tricuspid valvular intervention, which is sometimes difficult to visualize with 3D TEE, fusion imaging techniques have been a valuable aid for marking reference structures in successful procedures.38,39 In truth, considering the cost and time implications of implementing the technique, it will probably be more beneficial to use it selectively in complex cases, which would give a more beneficial cost-effectiveness ratio.
On the other hand, we have observed a simplification of more frequent procedures, such as TAVR (fluoroscopy alone, conscious sedation without intubation or use of TEE). In these settings, the dynamic modalities probably have little added value. However, static methods (fusion with CT) may be valuable to improve the accuracy of valve implantation, with published results suggesting a reduction in contrast dose. It should also be noted that it would be interesting to expand the co-registration technology of fluoroscopy with CT to facilitate vascular access, a key step in procedural safety.29 In line with the results of a German proof of concept study,32 static fusion imaging technology with 3D CT reconstruction enables guided femoral vascular access, as well as assisting in the remaining procedural steps, with benefits in terms of radiation and contrast doses. If subsequent studies emerge to prove this method is not inferior to ultrasound-guided access, the use of ultrasound could be avoided in procedures guided by fusion imaging.
However, some limitations must be noted. Firstly, an error of one to two milimeters has been reported during co-registration, which can naturally compromise the intervention. The limitations of co-registration are higher in the static modalities (possible incorrect alignment of the two images, non-compensation of cardiac and respiratory movements). However, this limitation can be mitigated with adequate technique and constant technological improvement.10 Secondly, the difficulty in assessing some anatomical structures persists, notably the pulmonary valve, which is particularly important in congenital heart disease. Finally, despite some authors claiming fusion imaging is more beneficial in teams with less clinical experience,37 use of this technology is associated with a considerable learning curve, which can impact on interventions.5,10,12,27,34 The results currently available on the benefits of fusion imaging may be underestimated, as it is a recent technology. Furthermore, there are few studies that have found it to be superior to the gold standard of percutaneous interventions. In fact, many of the works cited in this review have a retrospective and observational methodology, with significant sources of bias, thereby limiting them in terms of efficacy and safety analysis. There is an unquestionable need for randomized, prospective, multicenter studies, with adequate weight and sample size, which assess the clinical benefits and cost-effectiveness of fusion imaging. As the results are expected to be better in interventional teams that have more experience with this technology, the operators’ experience should be taken into account in the study design and analysis.
Fusion imaging has considerable development potential. As well as the clinical applications referred to in this review, some authors argue it has possible benefits in other interventions, such as endomyocardial biopsies or implantation of resynchronization devices.10,37
The literature also refers to 3D model printing and 4D projections as promising tools for percutaneous intervention planning, particularly in the most complex cases and in coronary interventions,40 among others. These methods will garner particular interest if they are able to simulate the tissue deformation that occurs during procedures. However, they are not capable of integrating cardiac movement and are associated with significant costs, making a good cost-benefit analysis necessary.1,41
ConclusionFusion imaging has substantial development potential in interventional cardiology and initial evidence suggests a possible reduction in procedure time, radiation dose and contrast volume.
Conflicts of interestThe authors have no conflicts of interest to declare.