One of the greatest challenges in medicine consists of arriving at a correct diagnosis despite different presentations of the disease. We present a case in which, notwithstanding the initial diagnosis, the search for the etiology was essential for clinical guidance. Left ventricular non-compaction (LVNC) was first described by Chin et al. in 1990. This relatively new entity is characterized by excessive thickening of the myocardial wall, formed of a thin epicardial layer and a substantially thicker non-compacted endocardial layer. The clinical presentation is highly variable but it must always be borne in mind that heart failure, atrial and ventricular arrhythmias and embolic events are common complications of LVNC.
Um dos maiores desafios da medicina é a realização de um diagnóstico correto, apesar das diferentes possíveis apresentações de cada doença. Em seguida apresentamos um caso que, apesar de um diagnóstico inicial, a busca da etiologia foi fundamental para a orientação clínica. A não compactação do ventrículo esquerdo (LVNC) foi primeiramente descrita por Chin et al, em 1990. Esta patologia relativamente nova é caracterizada pelo espessamento da parede miocárdica, constituída por uma camada fina epicárdica e uma camada substancialmente mais espessa de miocárdio não-compactado na região endocárdica. A apresentação clínica pode ser altamente variável, mas é preciso sempre ter em mente que a insuficiência cardíaca, arritmias atriais e ventriculares e eventos embólicos são as complicações mais comuns de LVNC.
We present the case of a 65-year-old woman with known cardiovascular risk factors of hypertension, obesity and dyslipidemia, with no other relevant medical history. She had excellent functional capacity and reported only occasional episodes of palpitations, short and self-limited.
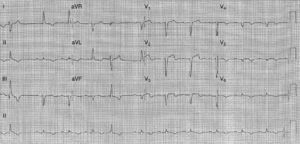
In the course of her normal daily activities she presented an episode of severe anterior chest pain after exertion, accompanied by breathlessness, sudoresis, and nausea. She was taken to our emergency room, where the admission ECG showed sinus rhythm, intraventricular conduction disturbance – incomplete left bundle branch block – and ST-segment elevation in V1–V3 (Figure 1). In the emergency room her clinical condition started to deteriorate with marked hypotension and alteration of consciousness. The physical examination revealed no relevant alterations.
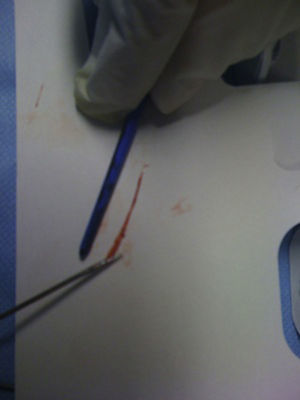
She was immediately taken to our catheterization laboratory, where coronary angiography showed occlusion of the distal left descending coronary artery, with no other lesions in the coronary tree. Thrombus aspiration was performed, with immediate establishment of TIMI 3 flow, and no stenosis was observed. The thrombus appeared highly organized (Figure 2), complementary in shape to the coronary artery. No atherosclerotic lesions were observed despite the patient's age and cardiovascular risk factors, which was highly suggestive of myocardial infarction without obstructive coronary disease. Further investigation was indicated to clarify the etiology.
She was admitted to our coronary care unit after the procedure and evolved favorably in Killip class I; peak troponin I was 38ng/ml. Hemodynamically she gradually improved, responding well and tolerating therapy with low-dose beta-blockers. Telemetric ECG monitoring documented short periods of atrial fibrillation and persistence of left bundle branch block. There were no more episodes of chest pain.
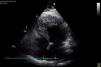
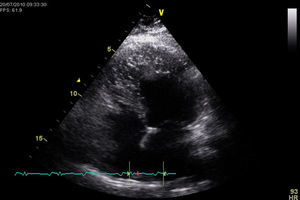
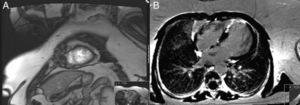
The transthoracic echocardiogram revealed a normal-sized left ventricle with moderate systolic impairment and hypertrabeculation of the left ventricular apex and hypokinesia of the anteroseptal wall and apical region (Figure 3). Doppler color flow mapping confirmed flow between the trabeculations. Cardiac magnetic resonance imaging (MRI) confirmed left ventricular hypertrabeculation, mainly in the apical and mid segments, with moderate systolic dysfunction. Transmural apical late enhancement was also evident, compatible with myocardial infarction (Figure 4).
These findings were suggestive of left ventricular non-compaction; this case presents the three major manifestations of the disease that ultimately resulted in coronary artery occlusion, precipitating an acute coronary syndrome.
She was discharged on the 8th day, referred to the cardiology clinic and medicated with bisoprolol, lisinopril, simvastatin and warfarin.
DiscussionThis case is a good example of the search for the etiology of a disease leading to the discovery of an association with a completely new condition, which requires different management. Cardioembolic events are not an uncommon manifestation of LVNC but presentation as an acute coronary syndrome is.1,3 Such embolic events are related to the presence of deep trabeculations in the ventricular walls, depressed systolic function and atrial fibrillation that predispose to thrombus formation. The incidence of stroke, pulmonary embolism and mesenteric infarction has been reported as 21–38%.
Various ECG abnormalities occur in LVNC, including ST-segment depression, negative T-waves, bundle branch block, pathologic Q-waves and atrial arrhythmias; there is no specific ECG pattern in this disease. The arrhythmogenic potential of LVNC is very high, thought to be due to subendocardial fibrosis resulting from microcirculatory dysfunction that is not confined to the non-compacted segments. Atrial fibrillation was another manifestation of this disease in this patient and there is also a high incidence of patients who develop paroxysmal supraventricular tachycardia and complete heart block. Recent studies report malignant ventricular arrhythmias in 47% of symptomatic patients and sudden cardiac death (SCD) has been reported in 13–18%.7 Annual 24-hour Holter monitoring is indicated and anticoagulation should be considered; an implantable cardiac defibrillator (ICD) may be used as an early therapy given the risk of SCD. A report by Kobza et al.8 showed that in 8 of 12 patients with LVNC (followed for a median of 36 months) who were treated with an ICD for secondary prevention, appropriate ICD therapy occurred in 50% of cases. In contrast, an appropriate ICD therapy was only documented in 25% of cases with primary prevention. The fact that supraventricular arrhythmias were documented in two-thirds of patients with an ICD has important implications for management strategies and the need to select devices with reliable detection enhancements when ICD implantation is considered. The role of biventricular pacemakers in this population remains unclear.
Two-dimensional and color Doppler echocardiography remain the gold standard diagnostic tools as they enable visualization of the ventricular trabeculations and their morphology. Cardiac MRI is complementary to the echocardiographic examination and the diagnostic criteria can also be applied. Jenni et al.2 described diagnostic echocardiographic criteria for LVNC, namely: (1) the absence of coexisting cardiac abnormalities; (2) a two-layered structure of the ventricular wall with an end-systolic ratio of the non-compacted to the compacted layer >2; (3) the finding of this morphologic presentation in apical and mid-ventricular areas; and (4) direct blood flow from the ventricular cavity into the deep intertrabecular recesses, as assessed by color Doppler echocardiography. MRI and myocardial perfusion scanning6 can be of additional help in detecting subendocardial perfusion defects. A recent MRI study concluded that a ratio of non-compacted to compacted myocardium greater than 2.3 would reliably identify the disease. It has recently been reported that the regular use of cardiac MRI may improve detection of LVNC.
The American Heart Association's 2006 classification of cardiomyopathies considers LVNC as a genetic cardiomyopathy. Nomenclature in this disease is still a matter of debate; biventricular non-compaction has been reported, while right ventricular non-compaction is also described in less than one-half of patients.4,5 The true prevalence of LVNC remains unknown; it is frequently associated with neuromuscular disorders. Mutations in LDB3 have been described in patients with this cardiomyopathy, and so routine echocardiographic screening of first-degree relatives is recommended.
ConclusionsThe diagnosis of LVNC in our patient was made after a myocardial infarction interpreted as an embolic event. She presented moderate left ventricular systolic dysfunction and echocardiography and cardiac MRI confirmed the diagnosis. She had a history of palpitations and short asymptomatic periods of atrial fibrillation were documented. She was started on anticoagulation, beta-blockers and angiotensin-converting enzyme inhibitors. As an outpatient she remains in NYHA class II and no further arrhythmic or embolic events have been reported to date. This case presents the three major manifestations of LVNC that ultimately resulted in coronary artery occlusion, precipitating an acute coronary syndrome.
Conflicts of interestThe authors have no conflicts of interest to declare.