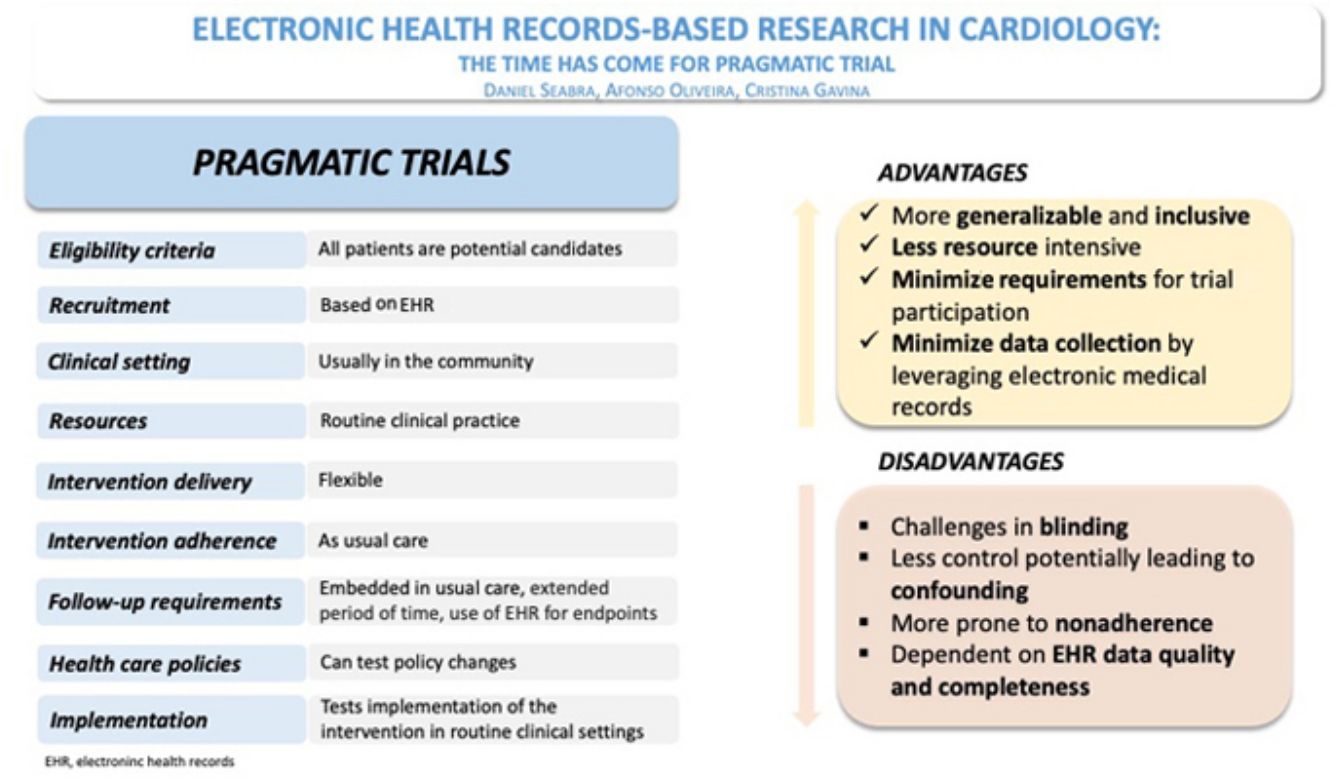
Randomized clinical trials (RCTs) are the cornerstone of evidence-based medicine, as they minimize bias in the allocation of interventions. However, RCTs performed in a very selective population and overcontrolled conditions may impair the generalizability of results. Moreover, increasing running costs and regulatory complexity compromise the conduct of these studies. The need for pragmatic trial designs, with streamlined procedures and low running costs, will shape the short-term future of research in RCTs.
Electronic health records (EHR) are routinely collected as part of the treatment of patients. These provide large amounts of data at no significant cost. The so-called “real-world data” are often used in observational studies with unavoidable bias. However, by combining the randomization of large numbers of patients with the data collected in EHRs, it is possible to answer very relevant clinical questions at a relatively low cost.
In this review, we describe how the integration of EHR and randomization is fostering innovative approaches to the conduct of RCTs in Cardiology.
Os ensaios clínicos randomizados (ECR) são o fundamento da medicina baseada na evidência, garantindo que o efeito observado de uma determinada intervenção depende apenas da alocação das intervenções. Contudo, os ECR são geralmente conduzidos em populações muito selecionadas e em condições extremamente controladas, o que pode limitar a generalização dos seus resultados. Além disso, os elevados custos e a complexidade regulamentar associada aos ECR representam barreiras significativas à sua execução. A necessidade de ensaios pragmáticos, com procedimentos simplificados e custos operacionais reduzidos, está a moldar o futuro da investigação em ECR.
Os registos de saúde eletrónicos (RSE), realizados por motivos clínicos ou administrativos, oferecem uma vasta quantidade de dados sem custos adicionais significativos. Os «dados do mundo real» são frequentemente utilizados em estudos observacionais, mas estes, embora revelando associações e levantando hipótese de estudo, têm como maior limitação a existência de fatores confundidores desconhecidos, para os quais não é possível fazer ajustamento. A combinação da randomização de um grande número de doentes com os dados recolhidos através dos RSE permite responder a questões clínicas pertinentes a um custo significativamente mais baixo e com menor sobrecarga das equipas de investigação e dos participantes.
Esta revisão descreve como a integração dos RSE e da randomização está a promover abordagens inovadoras na realização de ECR pragmáticos em Cardiologia, potencialmente revolucionando a forma como esses estudos são conduzidos e melhorando a sua aplicabilidade clínica.
Randomized clinical trials (RCTs) are the gold standard for clinical research in evidence-based medicine. Nevertheless, “traditional” or “proof-of-concept” RCTs determine the efficacy and safety of an intervention under ideal conditions, challenging their generalizability in clinical practice. They are becoming increasingly more complex and expensive, due to the need for large sample sizes and laborious procedures, endangering the survival of this model of knowledge generation.1
Nowadays, most of the studied treatment strategies for chronic diseases are expected to have only moderate effects since the pathophysiology is often multifactorial and the interventions focus only on a few pathways to avoid off-target effects. This requires including thousands of participants and following them up for extended periods to have the adequate statistical power for testing “hard” outcomes. Trial activities require substantial resource allocation from sponsors, investigators, and patients, thus limiting investment in less profitable research areas and slowing the pace of evidence generation.2,3 Moreover, regulatory approval requires compliance with a series of procedures that have been increasingly more complex. They have become the core business of contract research organizations, exponentially raising costs. Hence, RCTs are becoming amenable to only a small number of large pharmaceutical companies and are designed to answer a small number of specific questions, many of which are of questionable clinical relevance.
To circumvent the current limitations of traditional RCTs, investigators endeavor to find new evidence-generation options that can guarantee scientific rigor and patients’ safety in a more effective manner.4
Routinely collected electronic health records in researchRoutinely collected electronic health data (EHR) consist of health-related information that is collected and made available regularly in a standardized manner. It is often collected for billing, planning or administrative reasons, but it can be repurposed for health research. This information differs from that collected to address a specific research question, without the intention of regularly repeated assortment. Examples of EHR are birth and mortality records (vital statistics), data from primary and secondary care on diagnosis, procedures, and medications (health services data), disease-specific registries, and a range of national or regional health surveys and audits.5
Electronic health records are a repository of large amounts of information and an appealing source for research. Nevertheless, their use can be misleading since non-randomized analysis of “real-world” observational data can be used for hypothesis generation but only randomization can overcome both reported and unknown bias, regardless of the number of patients being examined.1 In an environment with randomization, EHR can be very efficient in participant identification and recruitment, outcome assessment and follow-up for legacy effects and long-term safety, making very large and well-powered trials, looking for modest effects, feasible and affordable to academics.
The use of EHR for research has several advantages. Data are readily available, at a low cost, with a lower risk of missing data. This reduces the burden for on-site staff and participants in screening and follow-up.
Despite their potential advantages, EHR data have been used in a small number of trials. There are several challenges for their widespread use due to national and local differences in data access and availability, uncertainty about data quality and inexperience in the process of approval, database linkage and data management.5 Fortunately, many regulators are issuing quality guidance for trials based on routine collected data, such as the Consolidated Standards of Reporting Trials (CONSORT) statement for trials conducted using cohorts and routinely collected data (CONSORT-ROUTINE),6 which have helped to clarify and standardize processes.
However, since EHR are not often collected with a clinical research question in mind, their usefulness will depend on the availability of relevant information for the population of interest, accuracy and reliability of the reporting mechanism, and timeliness of reporting. There are also local issues related to regulatory approval and ethics that can compromise their use. Accessing and sharing data will depend on national and regional policies, potentially compromising multicenter and multinational trials. Additionally, harmonization of information systems and data storage and management, especially if datasets are very large, can be challenging and require specific expertise and budget adjustments.5
To overcome some of these limitations, several countries are building health information networks, such as the US National Patient-Centered Clinical Research Network (PCORnet),7 the UK NHS Digital8 and the European Health Data and Evidence Network (EDHEN),9 to harmonize health records into a common data model, allowing for the merging of databases. These have demonstrated the feasibility of using EHRs for clinical research, particularly in comparative effectiveness trials.10,11 With a similar objective, registries have progressively been repurposed as platforms for randomized trials, as is the case of the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART)12 and the UK Myocardial Ischemia National Audit Project (MINAP).13
Use of electronic health records in clinical trialsIn pragmatic trials, EHR can be used in all stages of trial design and conduct. Information can be effectively collected from routine EHR, using disease coding and general demographics to identify suitable participants, and procedures and hospital admission data for outcome assessment and safety data.
But these trials require extensive planning and anticipation of several challenges. To be useful, data collection using EHR should be unbiased (unaffected by knowledge of the treatment allocation), complete (collected on all participants irrespective of whether they continue with the intervention), accurate, timely (up to date) and feasible (must be affordable within trial budget and feasible for participants and available in the relevant region).
The use of EHR has the enormous potential of reducing costs and increasing the efficiency of RCTs. Investigators must be aware of any possible caveats. Relying on routinely collected EHR for follow-up and outcome assessment might increase the risk of missing data or events. A thoughtful monitoring process must be in place to minimize such risks.
Trial designRoutine healthcare data can effectively inform protocol design and enable novel trial designs. Nonetheless, it poses limitations with regard to the questions that can be addressed, the design that can be implemented and the outcomes that can be evaluated.
Electronic health record data are particularly useful for simple and streamlined trial designs, using data focused on hard outcomes, with no centralized event adjudication and off-site monitoring. This data source is not suitable for all types of trials, particularly when safety is a concern or outcome assessment is dependent on independent adjudication. Therefore, the use of EHR is not suitable for phase 1 and 2 trials, mechanistic or proof-of-concept trials, and phase 3 trials with an investigational medicinal product – drug or device – where detailed information on safety, efficacy or pharmacodynamics is procured. Comparative effectiveness trials are a good example of EHR application, focusing on optimizing health outcomes by randomly comparing relative effectiveness of existing interventions in usual care to generate high-quality evidence to inform clinicians, healthcare providers, policy makers and patients.14
Randomized clinical trials embedded in routine clinical practice (Table 1) and data collection are an example of pragmatic and streamlined evidence generation and can provide three major benefits: (1) increasing value through better feasibility (reducing costs, time, and resources); (2) expanding the research agenda, i.e. performing trials for research questions of academic interest; and (3) offering novel design and data collection options.
Examples of clinical trials that used EHR-based research.
| ADAPTABLE(Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness) | Study design: Randomized, parallel, open-label.Objective: Evaluate aspirin 81 mg compared with 325 mg among patients with established atherosclerotic cardiovascular disease (ASCVD).Population studied: Patients with established (ASCVD): prior myocardial infarction (MI), prior coronary revascularization, prior coronary angiogram with ≥75% coronary stenosis, or history of chronic ischemic heart disease, coronary artery disease, or ASCVD.Outcomes: Primary effectiveness outcome: all-cause death, MI, or stroke at 12 months. Primary safety outcome: major bleeding requiring blood transfusion at 12 months. |
| REVeAL-HF(Risk EValuation And its Impact on ClinicAL Decision Making and Outcomes in Heart Failure) | Study design: Randomized, controlled, completely electronicObjective: Test an electronic alert system that informs practitioners about their hospitalized heart failure (HF) patient's 1-year predicted mortality using validated data from the HER.Population studied: Patients hospitalized for HF (an NT-proBNP levels of >500 pg/ml and receive intravenous diuretic agents within 24 h of admission).Outcomes: Primary outcome: composite of 30-day hospital readmissions and all-cause mortality at 1 year. Secondary endpoints: length of stay, discharge doses of HF therapies, palliative care referral, referral to electrophysiology and referral for advanced HF therapies. |
| HiSTORIC(High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction) | Study design: Randomized, controlled, stepped-wedge cluster.Objective: Determine the efficacy and safety of implementing an accelerated pathway where high-sensitivity cardiac troponin testing is used to rule out MI at presentation in consecutive patients with suspected ACS.Population studied: Patients presented to the emergency department or acute medical receiving unit with suspected ACS and had a high-sensitivity cardiac troponin I concentration within the normal reference range (less than the sex-specific 99th percentile upper reference limit) at presentation.Outcomes: Two co-primary endpoints – 1. Efficacy endpoint: length of stay from time of presentation until final hospital discharge. 2. Safety endpoint: free from type 1 or 4b MI or cardiac death from discharge to 30 days. |
| SSaSS(Salt Substitute and Stroke Study) | Study design: Cluster randomization, parallel, open-label.Objective: Evaluate a salt substitute compared with regular salt among patients with prior stroke.Used to rule out MI at presentation in consecutive patients with suspected ACSPopulation studied: Patients with prior stroke or risk for stroke.Outcomes: Primary outcome: stroke. Secondary outcomes: major adverse cardiovascular events (composite of nonfatal stroke, nonfatal acute coronary syndrome, or death from vascular causes) and death from any cause. Safety outcome: clinical hyperkalemia; sudden death. |
| RECOVERY(Randomized Evaluation of COVID-19 Therapy) | Study design: Randomized, open-label.Objective: Identify treatments that may be beneficial for people hospitalized with suspected or confirmed COVID-19.Population studied: Hospitalized patients with clinically suspected or laboratory-confirmed SARS-CoV-2 infection.Outcomes: Primary outcome: all-cause mortality. Secondary outcome: 1. Duration of hospital stay; 2. Composite endpoint of death or need for mechanical ventilation or ECMO. |
| TASTE(Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) | Study design: Randomized, parallel.Objective: Investigate whether aspiration thrombectomy as an adjunct to primary PCI resulted in superior outcomes as compared with primary PCI alone in patients presenting with STEMI.Population studied: ST-segment elevation MI undergoing primary PCI.Outcomes: Primary endpoint: all-cause mortality at 30 days. Secondary endpoints: 1. Time to rehospitalization with reinfarction at 30 days; 2. Time to stent thrombosis at 30 days. |
| DETO2X-AMI(Determination of the Role of Oxygen in Suspected Acute Myocardial Infarction) | Study design: Randomized, controlled, parallel-group, open-label.Objective: Evaluate supplemental oxygen therapy compared with ambient air among patients with suspected acute myocardial infarction (MI).Population studied: Patients with symptoms suggestive of MI (defined as chest pain or shortness of breath), an oxygen saturation ≥90% on pulse oximetry, and either electrocardiographic changes indicating ischemia or elevated cardiac troponin T or I levels on admission.Outcomes: Primary outcome: all-cause mortality at 12 months; secondary outcomes: 1. Rehospitalization with MI at 1 year; 2. all-cause mortality or rehospitalization with MI at 1 year. |
| ASCEND(A Study of Cardiovascular Events in Diabetes) | Study design: Randomized, parallel, factorial.Objective: Evaluate aspirin compared with placebo among diabetics with no known cardiovascular disease.Population studied: Patients with diabetes mellitus (any type) and without known cardiovascular disease.Outcomes: Primary efficacy outcome: major adverse cardiovascular events (vascular death, myocardial infarction, or stroke/transient ischemic attack). Primary safety outcome: major bleeding (intracranial hemorrhage, gastrointestinal (GI) hemorrhage, or sight-threatening eye bleeding). |
| TRANSFORM-HF(ToRsemide compArisoN With furoSemide FORManagement of Heart Failure) | Study design: Randomized, parallel, open-label.Objective: Evaluate furosemide compared with torsemide after hospitalization for decompensated heart failure (HF).Population studied: Patients hospitalized with worsening of chronic heart failure (HF), or new diagnosis of heart failure and meets one of the following criteria: left ventricular ejection fraction (EF) ≤40% and elevated natriuretic peptide level.Outcomes: Primary outcome: all-cause mortality. Secondary outcomes: All-cause mortality or hospitalization. |
Electronic health records can guide important decisions concerning trial feasibility, advising on patient and site selection. For example, data on the number of eligible patients, local expertise in procedures, local condition data management, conditions of data security and confidentiality can be obtained using EHR. The creation of electronic health data research networks has scaled up this process. A recent example is the ADAPTABLE Trial (Aspirin Dosing – A Patient-Centric Trial Assessing Benefits and Long-term),10 in which the PCORnet was used for recruitment, electronic consent and follow-up. Its open-label design could be a disadvantage, but routine health data are often collected by individuals not involved in the study and who are therefore blinded to the allocation of trial participants.
Outcome definition and assessment are an essential component of RCTs. Outcomes must be relevant for participants and occur at frequency high enough to guarantee appropriate statistical power. Moreover, their assessment should be unbiased and unequivocal. Therefore, when investigators consider using EHR for outcome assessment, they should make the necessary adjustments to ensure that the RCT goals are accomplished. In the ADAPTABLE trial,10 the primary outcome was not the traditional 3-point major adverse cardiovascular outcomes (cardiovascular mortality, nonfatal myocardial infarction (MI) and nonfatal stroke) but a more easily automatically collected composite of death from any cause, hospitalization for MI, and hospitalization from stroke. It is still clinically relevant and less subject to ascertainment bias.
The use of EHR offers the opportunity to obtain information for uncommon, but important, outcomes as the use of heart failure therapies or referral to palliative care, or the impact of information on prognosis on clinical decision making and outcomes, as in the innovative Risk EValuation And its Impact on ClinicAL Decision Making and Outcomes in Heart Failure (REVeAL-HF Trial).15
The non-adjudication of events may be a limitation to the use of EHR to assess outcomes, since their accuracy relies on high-quality records. Concerns about coding inaccuracies or bias introduced by selection of codes driven by billing incentives, rather than clinical care alone, may lead to the risk of misclassification bias or dilution effects. However, these effects may be offset by randomization and larger sample sizes.16,17
This model of data collection limits the chance of having more complex or specific outcomes unless these are collected separately. The High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction (HiSTORIC) trial had two co-primary endpoints, one collected using national registries and another pertaining to the type of MI that required independent adjudication.18
The use of EHR can equally be applied to traditional trial design and innovative methodologies. The ADAPTABLE trial,10 an open-label parallel arm trial is an example of the former, whereas the cluster RCT Salt Substitute and Stroke Study (SSaSS),19 the step-wedge cluster randomized trial (HiSTORIC) and the adaptative platform trial of Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial20 are examples of the latter.
In the first decade of the 21st century, the concept of registry-based RCTs (RegRCT) first emerged. These trials use the platform of an ongoing high-quality observational health registry as a case-report form for randomization and follow-up purposes. This design facilitates the randomization of a large number of patients over a short period of time, at reduced cost, and facilitates the follow-up of all eligible patients not enrolled in the study. Typical examples of RegRCT are the Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE)21 and the Determination of the Role of Oxygen in Suspected Acute Myocardial Infarction (DETO2X-AMI)22 trials, that used the SWEEDHEART Registry with a built-in specific randomization module.
Trial screening and recruitmentPatient recruitment for RCT is becoming increasingly difficult. Those in favor of trials aim to recruit participants that are simultaneously eligible for the study, willing to join and expected to be compliant with the intervention and follow-up. However, the choice of strict eligibility criteria and the trend to select only high-risk population for the outcome of interest (thus reducing sample size) often leads to problems of slow recruitment and may threaten the generalizability of results. Currently, there is a strong trend for sites to have low enrollment rates which increase trial duration and is associated with worse clinical results and higher rates of protocol discontinuation.23,24
When using EHRs for participant identification, investigators must promote simple eligibility criteria, easily and accurately identified in electronic databases, acknowledging the limitations that available information were not collected for research purposes and, thus, the need to adjust inclusion and exclusion characteristics to those existents in databases. EHRs can also help with invitations and screening. The main advantage is to implement algorithms that identify large numbers of eligible patients by running queries with specific codes in regional or national databases (computable phenotype). Following that, automatically sending invitation letters with pre-screening questionnaires can increase the efficiency of the entire process.
Examples of different successful recruitment strategies based on health records are the ASCEND11 and ADAPTABLE10 trials. ASCEND was designed and conducted by Clinical Trial Service Unit at the University of Oxford and used an innovative strategy of patient identification from electronic regional diabetes registers or general practices from around the United Kingdom; after which a screening questionnaire was sent by mail inviting people to participate, provide consent and self-report on eligibility criteria. In ADAPTABLE,25 there was no mandatory recruitment method and the most common recruitment approaches were remote or virtual contact via email, mailed letters, online messaging embedded within EHRs, or telephone calls using patient-centric recruitment material developed through collaboration with patient partners.
Informed consent must be sought, both for data use and for trial participation, and different methods must be applied when using routine collected data, according to trial design and context. In the SSaSS trial,19 villages for cluster randomization were recruited through a group consent process that involved the leadership of each village and the local county bureaus of health. In ADAPTABLE,25 every patient received a link to the trial web portal (through the various recruitment approaches) and a personalized access code, where they could consent using a web-based, electronic informed consent platform, developed with the patient partners, and customized locally and approved by the institutional review boards of each participating health system.
Follow-upTraditional trial protocols require detailed data collection and rigid on-site follow-up visits, including information, procedures and visits that are not part of routine clinical practice. These requirements make trials unfeasible and unappealing for site investigators, patients and caregivers, and limit follow-up duration.
The use of EHR may help in mitigating many of these limitations. Pragmatic trials, embedded in clinical practice, may use EHR from routine visits for follow-up data collection, reducing the burden on sites and participants. Moreover, it can enable assessment of the long-term treatment effects, especially legacy effects, since it makes long-term follow-up feasible at low cost, with less missing data and participants lost to follow-up. It can also provide information on those included in the trial but also on the remaining population. Linkage with national registry of mortality was used for the evaluation of the primary outcome of all-cause death in the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial,20 and in the TASTE trial21 the National Swedish Coronary Angiography Registry was successfully used to follow-up participants. Other examples are the SSaSS19 and the ToRsemide compArisoN With furoSemide FORManagement of Heart Failure (TRANSFORM-HF) trial.2
One possible limitation in these studies is the assessment of adherence and lower opportunities of engagement with study population. Nevertheless, in the ADAPTABLE trial,25 it was possible to involve patients in trial design and assure adherence through online or telephone questionnaires.
Follow-up data collection using EHR can have several models. There are trials where a combination of information collected in a specific clinical report form linked to national registries can help to detect clinical events such as death, myocardial infarction and revascularization. This was the case in the SORT OUT interventional trials from the Danish research network.26,27 These trials enabled the detection of systematic outcomes without additional patient visits and then the events were independently adjudicated. In the ADAPTABLE trial,25 patients were contacted via the on-line patient portal, or telephone calls for non-internet participants, instead of traditional on-site visits.
One of the major concerns in the use of EHR for follow-up is the timeliness of reporting. Trial data must be collected at a clinical encounter and must be registered, processed, and made accessible for analysis. Up-to-date outcomes and safety reporting are paramount for Data Monitoring Committee analysis and for opportune dissemination of results and publication soon after the follow-up period ends. This can be surpassed using a hybrid approach, with additional telephone calls or outpatient visits, although this may require substantial resources. The use of emerging technologies using artificial intelligence and large language models may be of great help in making this process more efficient.
Ethical issues in pragmatic trialsAs previously mentioned, pragmatic trials can provide results that are more reflective of real-world practice that are applicable to real-world decisions. Nevertheless, new ethical issues may arise considering their streamlined design.28
One of the most relevant ethical points pertains to informed consent.29 It can be challenging to ensure that participants fully understand the nature and implications of the trial, especially when the study design is complex. Moreover, obtaining traditional written informed consent may be burdensome, and have a negative impact on recruitment, undermining the pragmatic objective of the trial.30 To address this point, there are alternative approaches, such as expanded use of waivers of consent31 especially for low-risk trials, or alternative approaches to standard written consent, such as electronic informed consent (E-consent), which involves the obtaining of consent via electronic methods.32 E-consent has particularly relevant advantages, as it allows expedite recruitment processes (including remote ones), maximizing accessibility to research opportunities and simplify monitoring. The establishment of a dynamic and longitudinal relationship between participants and the research team allows personalization of the information provided to the participant, according to their needs and preferences, with inherent gains in process efficiency for the research team involved in pragmatic clinical trials.33
Pragmatic trials present other ethical challenges due to their real-world settings and focus on generalization. It is essential to guarantee equity in access to participation by ensuring broad representativeness, with an assertive balance between the risk and benefit of a given intervention. In this type of trials, the line between clinical practice and research can blur, which can influence patient safety.34
Addressing these ethical issues requires careful planning, adherence to ethical standards, continuing communication with participants, and, where possible, involvement of stakeholders throughout the trial process.
ConclusionElectronic health records have considerable potential for improving the conduct and reducing the costs of RCTs, but understanding the methods used to collect routine data is essential to identify potential biases. Future research using these tools will require data quality validation, alternative research designs and ethical and regulatory improvements, as well as active involvement of patients.
Conflicts of interestThe authors have no conflicts of interest to declare.