In Portugal, a 15.2% prevalence of heart failure with preserved ejection fraction (HFpEF) was recently identified among those aged ≥50 years. HFpEF represents 90% of Portuguese heart failure patients.
HFpEF management in Portugal is challenging due to patient heterogeneity, diagnostic and therapeutic complexity, and organizational constraints on the healthcare system.
Considering the above, a panel of Portuguese experts convened to address HFpEF management within the national context. This was done in a two-paper set.
This, the second paper, identifies unmet needs and suggests a set of measures to improve the current organization of HFpEF management in Portugal. Our purpose is to create a multidisciplinary integrated care system, ensuring a seamless connection between hospitals and primary care. Additionally, we propose a practical approach to the management of HFpEF, including a roadmap for screening, diagnosis, referral and treatment. The aim is to help clinicians improve HFpEF management throughout the disease trajectory.
Em Portugal, foi recentemente identificada uma prevalência de 15,2% de insuficiência cardíaca com fração de ejeção preservada (ICFEp) em indivíduos ≥50 anos. A ICFEp representa 90% dos doentes portugueses com insuficiência cardíaca (IC).
A gestão da ICFEP em Portugal é um desafio devido à heterogeneidade dos doentes, à complexidade diagnóstica e terapêutica e às restrições organizacionais do sistema de saúde.
Considerando o acima exposto, reuniu-se um painel de peritos portugueses para abordar a gestão da ICFEP no contexto nacional. Este foi feito num conjunto de dois artigos.
Este segundo artigo identifica as necessidades não satisfeitas e sugere um conjunto de medidas de melhoria para a atual organização da gestão da ICFEp em Portugal. O objetivo é criar um sistema de cuidados integrados multidisciplinares, garantindo uma ligação perfeita entre os hospitais e os cuidados de saúde primários. Além disso, propomos uma abordagem prática para a gestão da ICFEp, incluindo um guião de rastreio, diagnóstico, encaminhamento e tratamento. O objetivo é ajudar os médicos a melhorar a gestão da ICFEP ao longo da trajetória da doença.
It is commonly accepted that heart failure with preserved ejection fraction (HFpEF) affects approximately 50% of patients with heart failure (HF).1 The incidence of HFpEF increases sharply with age, and 70% of HFpEF patients are aged >65 years.2 The prevalence of HFpEF is expected to rise in the near future due to aging of the population and the increasing prevalence of obesity, hypertension, metabolic syndrome, coronary artery disease and diabetes.3
The management of HFpEF can be challenging due to the heterogeneity of patient profiles and diagnostic and therapeutic issues. This position paper consists of two articles (Part I and Part II). Part I includes a review of the HFpEF literature covering pathophysiology, clinical presentation, and clinical management. In the present Part II, we address the current status of HFpEF management in Portugal, identifying unmet needs, proposing a pragmatic approach to HFpEF management, and presenting a roadmap for diagnosis and referral, along with a strategy for treatment implementation.
The prevalence of heart failure with preserved ejection fraction and associated burden in PortugalIn 2002, the EPICA study4 established an HF prevalence of 4.36% among the mainland Portuguese population, amounting to 400000 patients, of whom approximately 40% had preserved systolic function.4 The recent Portuguese Heart Failure Observational Study (PORTHOS), a population-based, nationwide, three-stage screening study in people ≥50 years of age that recruited over 6000 participants in mainland Portugal, updated these figures.5 According to this study, HF prevalence underwent a major increase in Portugal, reaching 16.5%, higher in older people (10.6 times higher in those aged ≥70 vs. 50–59 years) and in women (2.3 times higher). This was mainly due to a disproportionate increase in HFpEF, with a prevalence of 15.2%, about three quarters of whom were women and aged ≥70 years.6
Profile of patients with heart failure with preserved ejection fractionThe typical HFpEF patient profile is an elderly obese woman with multiple comorbidities (such as diabetes, hypertension, atrial fibrillation and chronic kidney disease), frequently living alone.7–11 These patient characteristics were also found in the PORTHOS HFpEF population.6
Portugal has one of the highest rates of elderly people in the world (23.8% of the Portuguese population in 2022).12 In 2021, nearly 450000 Portuguese people aged ≥65 years lived alone.13 Nearly 31% of PORTHOS participants aged 70 or older had HFpEF.6
Additionally, PORTHOS found a prevalence of 30.5% for obesity, 25.4% for type 2 diabetes (T2D), and 76.0% for hypertension in HFpEF patients aged ≥50 years.6
Overall, these data confirm a high burden of HFpEF in Portugal and many unmet needs regarding its management in the national context.
Heart failure with preserved ejection fraction in Portugal: unmet needsAccess to primary careComprehensive primary healthcare coverage is essential to diagnose and monitor health conditions such as HFpEF. However, 16% of the Portuguese population do not have a primary care physician under the National Health Service and, in many cases, even those who do, report limited access.14 Such limited access to primary care may compromise early HFpEF diagnosis, and constitutes a major unmet need in our national context.
Establishing the diagnosis of heart failure with preserved ejection fractionDiagnosing HFpEF can be challenging since numerous clinical conditions manifest with similar symptoms and signs. These include chronic respiratory diseases, obesity, venous pathology, and anemia. The presence of preserved left ventricular ejection fraction (LVEF) necessitates differential diagnosis with non-cardiac dyspnea, which occasionally requires advanced imaging or invasive cardiopulmonary exercise testing, particularly in patients who only develop physical manifestations of congestion during exercise.2,15,16
In recent years, integrated diagnostic approaches have been proposed based on composite scores derived from medical history, laboratory results, and electrocardiographic and imaging data.17–19 The European Society of Cardiology (ESC) heart failure guidelines state that a diagnosis of HFpEF should be based on the presence of typical symptoms and signs of HF, with additional information from natriuretic peptides, electrocardiography and echocardiography, to ensure efficient, early and accurate diagnosis.18
Natriuretic peptidesNatriuretic peptides are particularly important for diagnosing HFpEF, both as a rule-out test and as a marker of congestion, and for prognosis.18 However, in Portugal, access to this diagnostic test at the primary healthcare level remains limited due to a lack of reimbursement in this context.20–22
Interpretation of natriuretic peptide levels in HFpEF may not be straightforward, which underlines the need to train clinicians appropriately in the correct use and interpretation of the test. This is particularly important in patients with obesity, atrial fibrillation, advanced age or chronic kidney disease, conditions that carry a high risk of developing HFpEF and significantly impact natriuretic peptide levels.23
EchocardiographyThe situation throughout Portugal, in both primary healthcare and hospital settings, is of inequitable access to echocardiography, with long waiting lists in several hospitals.
There is considerable heterogeneity in the content of echocardiographic reports, with frequent omission of essential functional and structural parameters, which makes the inclusion of echocardiographic criteria in HFpEF diagnosis more challenging.
Additionally, in Portugal there is no reimbursement of Doppler modalities in primary care, so the great majority of patients in this context only have access to two-dimensional echocardiography.
Other cardiac structural and functional testsThere is limited access nationwide to comprehensive diagnostic work-up tests, such as cardiac magnetic resonance imaging and exercise stress tests with echocardiographic or invasive hemodynamic monitoring, that may be required to better identify etiology or to diagnose more challenging HFpEF patients.
Treating heart failure with preserved ejection fractionNon-pharmacological managementRegular exercise has been shown to offer a pleiotropic array of positive effects that can ameliorate cardiac and extracardiac abnormalities in HFpEF.24 Current guidelines strongly advocate for exercise training in HF patients, assigning it a class I recommendation (level of evidence A).24
Despite the potential of exercise to improve outcomes in HFpEF,24–26 and the growing number of cardiac rehabilitation centers in Portugal, there is still a shortage of cardiac rehabilitation programs and they are unevenly distributed throughout the country. In a 2019 survey, only 14.5% of referrals to phase II and 19.8% of referrals to phase III cardiac rehabilitation programs were due to HF, although the former percentage was higher than in previous surveys.27 There was a 37% decrease in patients undergoing phase III programs overall. These data reflect the need to increase the national coverage of cardiac rehabilitation programs and to overcome obstacles in the referral, enrollment, and adherence of HF patients. Additionally, other alternatives, such as cardiac telerehabilitation programs, should be adopted in view of their positive impact on functional measures and patients’ quality of life.28–31
Pharmacological therapyAs previously discussed,26 following the publication of the EMPEROR-Preserved and DELIVER trials, sodium-glucose cotransporter-2 (SGLT2) inhibitors have become a fundamental HFpEF therapeutic pillar with a class I indication, level of evidence A, in the ESC guidelines, alongside diuretics in patients with congestion.3
No data are available to date concerning the uptake of these medications in Portuguese HFpEF cohorts.
Managing comorbiditiesThe presence of multiple comorbidities in HFpEF patients is the rule, not the exception, adding to the complexity of their management and leading to an increased risk of pharmacological interactions.
The obesity phenotype is common in HFpEF.32 Weight loss can be achieved through lifestyle interventions including diet and exercise training, pharmacotherapy, and bariatric surgery.26
In Portugal, where 17% of the population is obese, there is asymmetry in access to obesity treatment, with most of the centers offering bariatric surgery concentrated in a few geographical areas, with different access to nutritional counseling and follow-up.33
Portugal does not have a pharmacological coverage strategy for obesity. A recent government directive sets out the implementation of an integrated care model for the prevention and treatment of obesity.34 However, this document deals with general principles and intentions, but lacks specific regulation and strategic operationalization.
Considering the available therapeutics, among glucagon-like peptide-1 agonists there are specific doses and formulations of both semaglutide and liraglutide approved in the EU for treating obesity, independent of T2D status, although they are not covered by the Portuguese National Health Service reimbursement system or by most health insurance plans.35 Accordingly, treatment costs may be a significant barrier to their use by obese patients in Portugal.
Hypertension, the most prevalent comorbidity in HFpEF, also constitutes a challenge in the Portuguese context. The INSEF study found that more than 30% of individuals aged between 25 and 74 years with hypertension were not aware of their condition, and 30% were not on medical treatment.3,36
There is still a considerable number of patients with unidentified atrial fibrillation. The SAFIRA study showed a 9% prevalence of atrial fibrillation in individuals aged ≥65 years, 36% of whom were unaware of their condition. Additionally, there are nationwide disparities in access to ablation therapy.
It is essential to implement strategies that identify and monitor high-risk patients, given the mechanisms that have been described underlying HFpEF and these conditions.26
Healthcare organizationThe PORTHOS study showed that HFpEF is more prevalent in women and in older individuals.6 Typical age-associated factors such as cognitive impairment, depression, reduced social support, frailty, and economic deprivation add a level of complexity to patient management. All these factors constitute serious barriers to access to appropriate therapy and to its adherence and optimization, and underline the need for a comprehensive approach.
Multidisciplinary teams are key structures for improving HF management, and may help overcome these barriers by providing patient-centered care to HFpEF patients.20 In Portugal, there are few examples of such integrated management, which is endorsed by international HF guidelines.18,19,37–40
Palliative care is another important component of HF management. Advanced age and a greater burden of comorbidities, compared to HF in patients with reduced ejection fraction, mean that HFpEF patients are particularly in need of this level of care. Palliative care units have been created in multiple hospital and primary healthcare centers all over Portugal. Nevertheless, few HF patients have access to palliative consultations in Portugal, typically only when approaching the end-of-life stage, probably due to many factors, including lack of awareness on the part of patients and providers and resource constraints.41–43 As is increasingly recognized, a palliative approach should begin early in the disease trajectory of HF patients, although not necessarily relying on formal palliative care teams.
Additionally, in order to improve HF care, there is a recognized need for health professionals to undergo specific training in HF, for HF care quality indicators to be defined, and for a national registry to be established.44
In conclusion, there is a large unmet need concerning planning and organization of HFpEF care across institutions of the Portuguese healthcare system, from individual primary healthcare and hospital centers to local, regional and national care networks.
Proposal for improving management of heart failure with preserved ejection fractionStakeholdersConsidering the aforementioned unmet needs, a national strategy aiming at improving HFpEF care should focus on the organization of a multidisciplinary integrated care system that is able to ensure a seamless connection between hospital and outpatient care.20 Additionally, support from social workers is paramount in reinforcing the connection between the health system and the patient's household.
In short, three levels of support of the National Health System should be considered: hospital services, outpatient health services, and community social support.20
In addition to the above-mentioned caregivers, this plan requires the active involvement of other stakeholders acting at different levels, including regulatory and political bodies (such as the Parliamentary Health Committee, the Ministry of Health, and the head of the Program for Cerebral and Cardiovascular Diseases).
Undoubtedly, a strong HF patients’ association, such as the Portuguese Association to Support Patients with Heart Failure (AADIC), is of paramount importance in creating the political leverage needed to implement this comprehensive HF care network.
The Portuguese Societies of Cardiology, Cardiothoracic and Vascular Surgery, Internal Medicine, General and Family Medicine, and Intensive Care, as well as the Portuguese Medical Pharmaceutical and Nursing Associations, should also be involved in this effort. The participation of the media is also essential to promote the public's and patients’ HF literacy, to enhance the visibility of HF and to leverage the efforts of the various HF stakeholders to improve HF care in Portugal. Finally, the pharmaceutical and medical devices industries can be important partners in supporting this effort.
This plan should follow the recommendations in the ESC HF guidelines and should include training programs for all caregivers involved.18
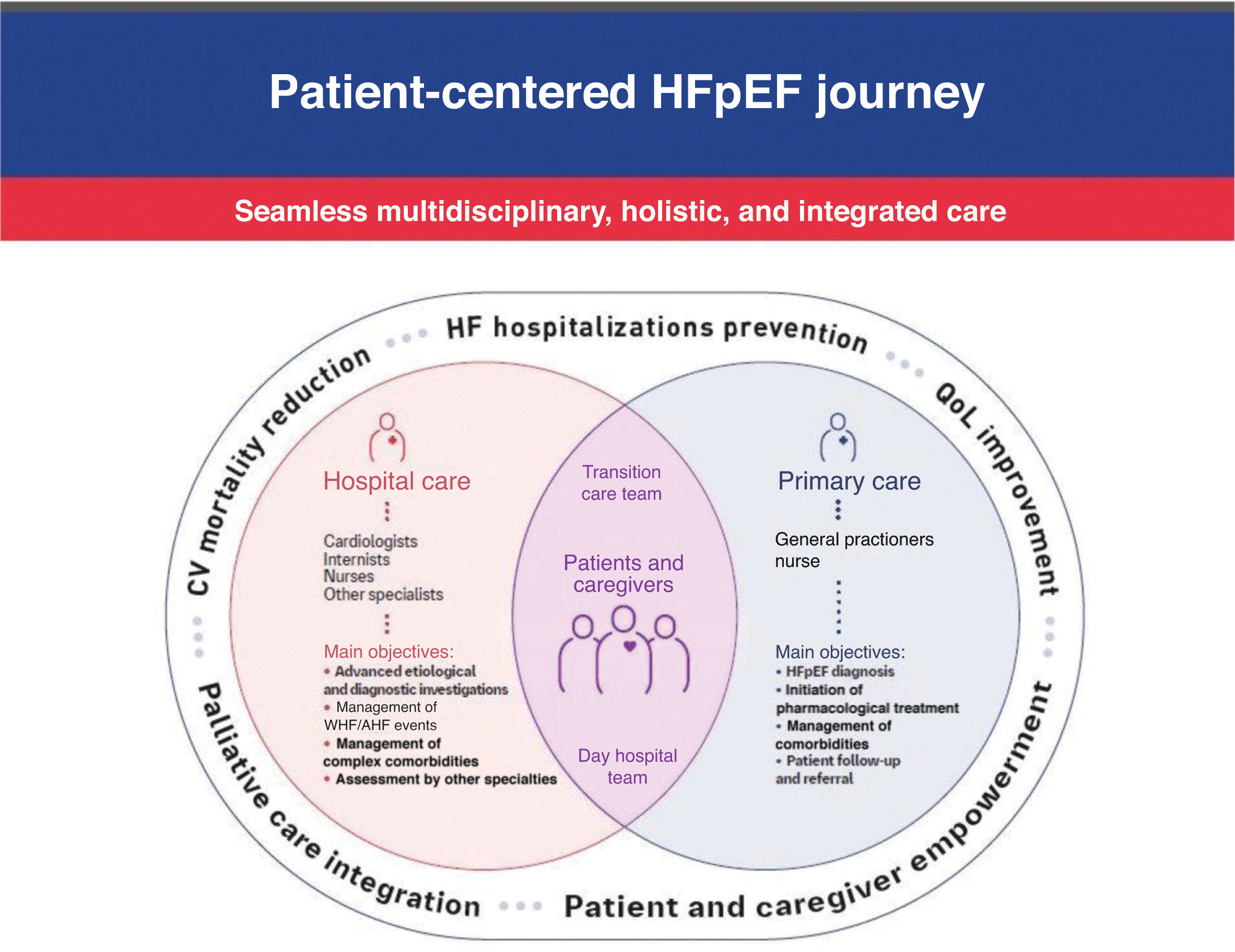
General strategyOrganization of care for HFpEF patients should consist in a unified system in which the patient, when stable, is managed in an outpatient context by primary care physicians and nurses, and when unstable, is managed by cardiologists and internal medicine specialists with the assistance of nurses in a hospital setting (Figure 1).20 Along this life journey, the patient and his/her informal caregiver should be viewed as a single entity, stressing the paramount role of informal caregivers in providing physical, emotional, and spiritual support.20
Primary care physicians should have a major role in establishing the HFpEF diagnosis, initiating non-pharmacological and pharmacological HFpEF therapy, promoting management of comorbidities, assuring patient follow-up, and referring HFpEF patients to hospital care when appropriate. Hospital specialists (cardiologists and internists) should be responsible for treatment of worsening HF and acute HF events, management of complex comorbidities and advanced diagnostic and etiological investigation, as well as assessment of patients’ eligibility for advanced therapies (Figure 1). Cardiology subspecialties may be needed to perform atrial fibrillation ablation (electrophysiologists) or to address coronary artery or valvular disease (interventional cardiologists and cardiac surgeons). Other medical specialists, such as endocrinologists, nephrologists and pulmonologists, may be needed to address relevant comorbidities.45 Additionally, psychiatrists and psychologists, palliative care practitioners, physiatrists and physiotherapists, and other professionals such as pharmacists, nutritionists, and social workers, may also be involved.
Settings of carePrimary healthcare and hospital settings should be integrated within a seamless communication system.20 Importantly, to strengthen this system, coordinated HF day clinic and HF transition care teams should be implemented to safely bridge the problematic patient transition from the outpatient setting to the hospital and vice versa (Figure 1).20
In cases of severe clinical instability, most patients are currently attended in the emergency department, with further admission to an intensive care unit or a cardiology or internal medicine ward. To reduce the hospital burden associated with frequent HF decompensations and to promote a more patient-centered approach, these decompensated patients should be attended at a HF day clinic and referred back to the outpatient setting after short-term administration of appropriate intravenous medication. This strategy enables more streamlined patient management, preferably within the local HF team network. A hospital-at-home strategy is suitable for some patients, particularly those with advanced HF and/or severe comorbidities and requiring intravenous diuretics, or after a short hospital stay.46,47 Following a Ministry of Health policy, Portugal has implemented several hospital-at-home units, generally led by internal medicine specialists, that provide substantial national coverage. HF is a common cause of admission, mirroring conventional hospitalization.48
An HF day clinic can provide short-term reassessment of early post-discharge patients, contributing to a safer and more efficacious transition strategy, enabling same-day blood analysis and more straightforward management of medical therapy, to replicate the successful STRONG-HF trial strategy.49
Thus, a circular system should be established, integrating the outpatient setting, HF day clinics, hospital admission, the transition care team and back to the outpatient setting, as a unified continuum of care (Figure 1).
Communication and networkingImportantly, this system should be based on a unified team approach. It should be built on ease of communication and sharing of information grounded in a common electronic record system. This should be reinforced by using a dedicated email address and/or telephone number and frequent teleconferences involving all the above in-hospital and out-of-hospital healthcare professionals, as well as members of transition care and HF day clinic teams.
These teleconferences should serve as a forum for discussing specific patient problems, as well as for sharing scientific knowledge. Other professionals should be encouraged to participate in these online meetings, so that they can also act as members of the multidisciplinary team.
Finally, this expanded multidisciplinary integrated care team should coordinate with other teams at a national level in order to create a ‘hub and spoke’ system.50 This will allow flexible communication between the periphery and more specialized centers, culminating in the few centers dedicated to advanced HF, in accordance with the ESC guidelines.50
An effective palliative care network should also be implemented,18 enhancing both the accessibility and the quality of palliative care for HF patients across the entire disease trajectory and not exclusively restricted to end-of-life care, by integrating palliative care into HF management earlier.51 General palliative care should be provided by HF specialists at different levels, with specialized input from the palliative care physician or specialist teams, as required.51
Quality monitoringPerformance indicators should be developed that assess outpatient and inpatient care to help improve quality.44
The development of a single, integrated, shared electronic health record dedicated to HF, combined with quality of care indicators, has been proposed to facilitate the capture of demographic, clinical, management and outcome data.44 An electronic platform is critical for launching a high-quality continuous clinical registry to determine compliance with guidelines and to develop programs for continuous quality improvement.52
Raising awarenessHF awareness campaigns, specifically addressing HFpEF, should be carried out targeting healthcare professionals, patients, and the general population.
The need for a pathway for heart failure with preserved ejection fraction in PortugalThe current fragmented and heterogeneous care delivered to HF patients in Portugal requires an effort to organize a streamlined structured pathway for the management of the syndrome. This need is particularly striking with regard to HFpEF, a condition with multiple diagnostic and therapeutic issues and uncertainties affecting a challenging patient profile.
A Ministry of Health policy on HF management has long been awaited and deemed extremely important. Nevertheless, medical societies should also have an active role in proposing measures to achieve the much-needed step forward in quality. Thus, we propose a pragmatic approach to the management of HFpEF patients in Portugal, including a roadmap for diagnosis, referral and treatment, based on international guidelines and adapted to the specific characteristics of the Portuguese situation.18,38 This is addressed to all healthcare professionals involved in the management of HFpEF patients.
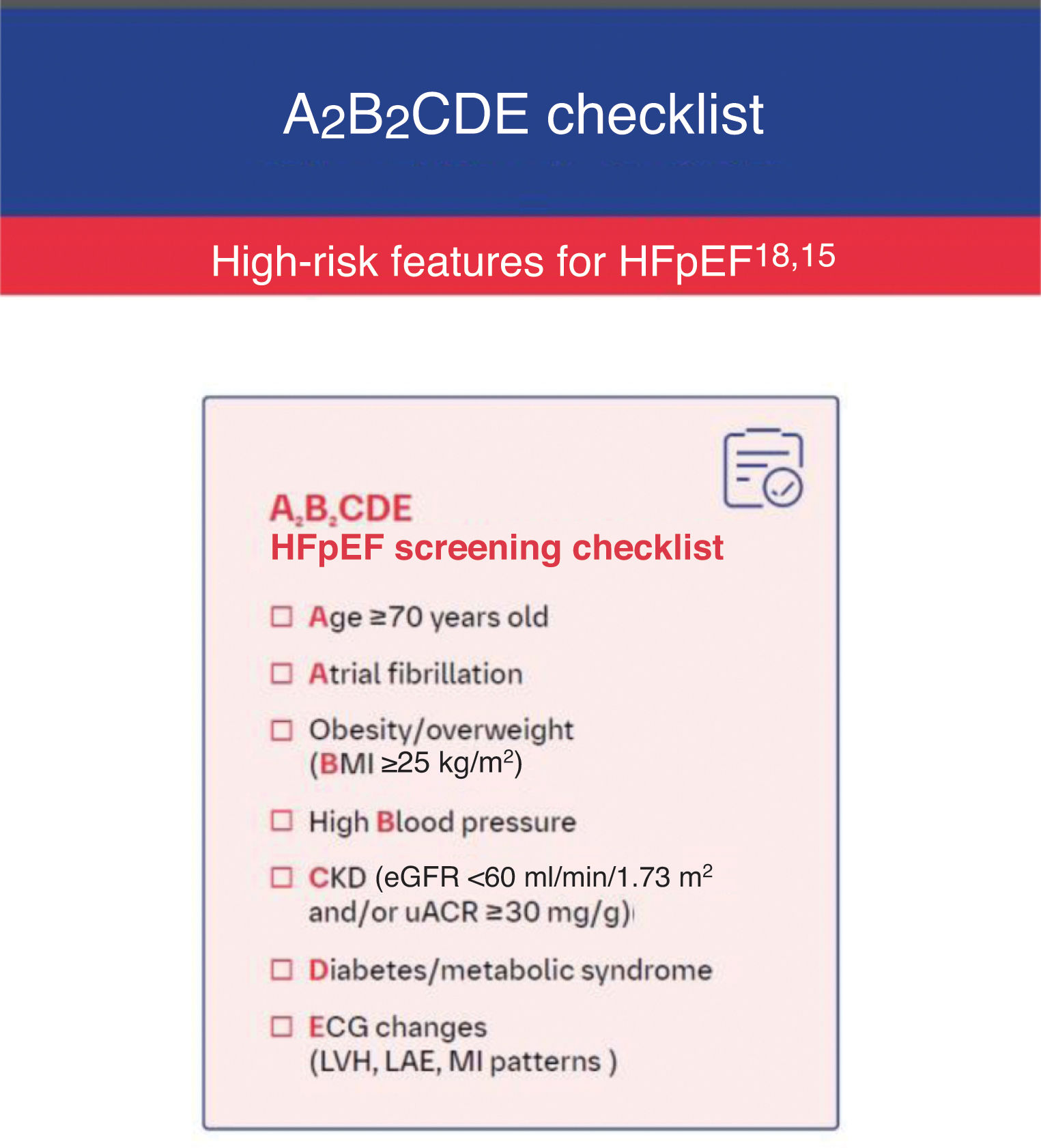
A pragmatic approach to management of heart failure with preserved ejection fraction in Portugal: a roadmap for screening, diagnosis, referral and treatmentDiagnosisSuspicion of heart failure with preserved ejection fractionSuspicion of HFpEF may arise in patients presenting with dyspnea and/or fatigue and/or peripheral edema. The concomitant presence of age ≥70 years, atrial fibrillation, obesity/overweight (body mass index ≥25 kg/m2), high blood pressure, chronic kidney disease, diabetes and/or metabolic syndrome, and electrocardiogram (ECG) changes increases the probability of HFpEF.
At this point, an ECG should be performed. If it identifies atrial fibrillation, left atrial enlargement and/or left ventricular (LV) hypertrophy, or myocardial infarction patterns, suspicion of HFpEF increases further.
In order to recall the above checklist, we propose ordering these seven characteristics according to the abbreviation A2B2CDE (Figure 2).
A2B2CDE checklist. BMI: body mass index; CKD: chronic kidney disease; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; HFpEF: heart failure with preserved ejection fraction; LAE: left atrial enlargement; LVH: left ventricular hypertrophy; MI: myocardial infarction; uACR: urine albumin–creatinine ratio. H2FPEF score available at: https://www.mdcalc.com/calc/10105/h2fpef-score-for-heart-failure-with-preserved-ejection-fraction.
HFpEF mimics such as thyroid, liver, pulmonary or advanced kidney disease, and anemia, should be excluded.
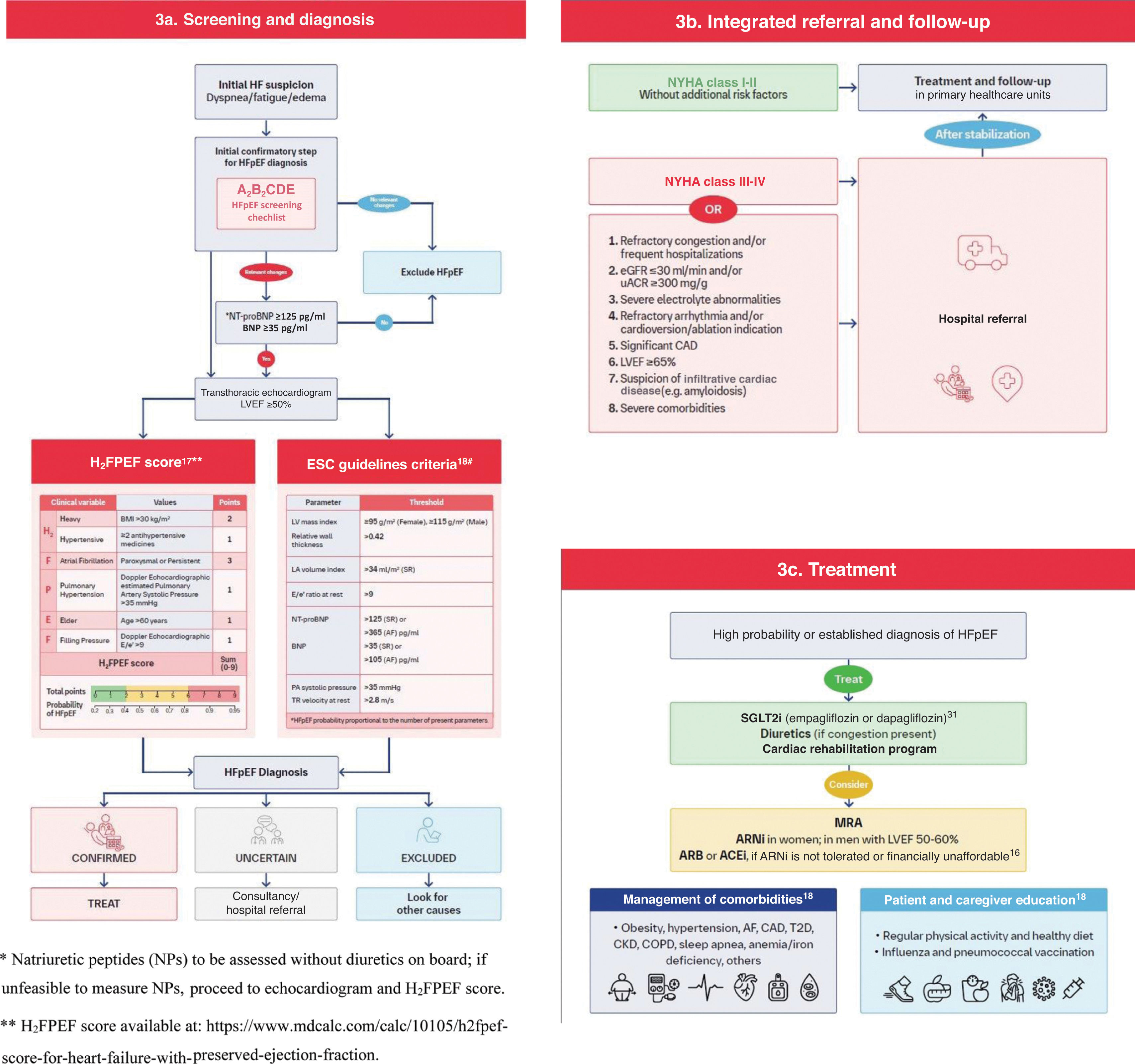
Natriuretic peptidesQuantification of natriuretic peptides (NPs) follows in the HFpEF diagnostic pathway. B-type natriuretic peptide (BNP) <35 pg/ml or N-terminal proBNP <125 pg/ml, in patients not treated with diuretics, renders HFpEF unlikely.53
If NP quantification is not available, echocardiographic assessment should follow directly (Figure 3a).
A roadmap for management of heart failure with preserved ejection fraction. (a) Screening and diagnosis; (b) integrated referral and follow-up; (c) treatment. ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; AF: atrial fibrillation; AMI: acute myocardial infarction; BMI: body mass index; BNP: B-type natriuretic peptide; CAD: coronary artery disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; LA: left atrium; LV: left ventricular; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro B-type natriuretic peptide; NYHA: New York Heart Association; PA: pulmonary artery; PASP: pulmonary artery systolic pressure; SGLT2i: sodium-glucose cotransporter-2 inhibitors; SR: sinus rhythm; T2D: type 2 diabetes; TR: tricuspid regurgitation; TTE: transthoracic echocardiography; uACR: urine albumin–creatinine ratio.
Echocardiographic examination, preferably including tissue Doppler, is central to the diagnosis of HFpEF. Quantification of LVEF is mandatory, as well as LV mass index, relative wall thickness, and left atrial volume index; these parameters can be readily obtained via two-dimensional echocardiography, which is widely available and reimbursed within the Portuguese primary healthcare system.
Doppler-derived variables that can be used to estimate LV filling pressures, including E/e′ ratio and pulmonary artery systolic pressure, are highly recommended. Alongside calculation of the H2FPEF score, we refer clinicians to pragmatic HFpEF diagnosis criteria derived from the 2021 ESC HF guidelines, in which Table 9 includes key echocardiographic variables, in combination with NPs, thus offering a consistent, yet simplified approach to the HFA-PEFF algorithm (Figure 3).53 Ideally, integration of all these parameters would improve HFpEF diagnosis in patients with LVEF >50%, as their combination increases the likelihood of HFpEF. However, echocardiographic reports often omit some of these variables. Thus, the specific inclusion of these parameters in echocardiographic requests is suggested.
Calculating the probability of heart failure with reduced ejection fractionAs there is no single non-invasive test to unequivocally diagnose HFpEF, diagnostic scores are clinically useful. Two clinical and echocardiographic scores have been developed for HFpEF diagnosis: the HFA-PEFF algorithm and the H2FPEF score.15,17 The HFA-PEFF algorithm is more complex than the H2FPEF score and includes more echocardiography-derived measurements.
Due to the high prevalence of HFpEF, most of these patients are attended at the primary care level. In this setting, the H2FPEF score appears to be more suitable than the HFA-PEFF algorithm, since it includes significantly fewer echocardiographic variables and has been hemodynamically validated.52 In a patient with suspected HFpEF, a score ≥6 is highly suggestive of the diagnosis.17,44 Additionally, there are several versions of the H2FPEF score calculators, both online and in smartphone apps, which may further support its dissemination in clinical practice (Figure 3a).
In addition to being more complex, the HFA-PEFF algorithm may in certain cases require invasive hemodynamic parameters, which are available in very few centers in Portugal.
Considering specific etiologies of heart failure with reduced ejection fractionEstablishing a HFpEF diagnosis requires investigation of specific etiologies that may present with HF signs and symptoms and preserved LVEF, but warrant distinct diagnostic and therapeutic management. Conditions like amyloid heart disease, hypertrophic or other restrictive/infiltrative cardiomyopathies, and valvular or pericardial disease should be considered during the diagnostic work-up, as established in step F2 of the HFA-PEFF algorithm.15,45 Although valvular disease is readily detected on a routine echocardiogram, other etiologies generally require more specific tests like cardiac magnetic resonance, invasive hemodynamic studies or cardiac biopsy that are only available in specialized hospital settings. Nevertheless, it is crucial for primary care physicians to suspect these conditions, integrating clinical assessment with clues from diagnostic testing (such as red flags for amyloid cardiomyopathy or LVEF ≥65%), to identify patients in need of hospital referral for etiological work-up and treatment.
Management strategy for heart failure with reduced ejection fractionWhenever an HFpEF diagnosis is confirmed (Figure 3c), an SGLT2 inhibitor should be prescribed.18,38 In cases of congestion, a loop diuretic should be added and subsequently titrated to the lowest dose to maintain euvolemia. Adding an MRA, particularly finerenone, may be considered, in light of the recent FINEARTS-HF topline results.54 For obese patients, semaglutide or tirzepatide may also be considered given the STEP-HFpEF and SUMMIT trials. It is expected that the increasing evidence and pharmacological therapeutic options will be reflected in future international guideline updates.
Patients in New York Heart Association (NYHA) class I-II without additional risk factors should generally be managed in the primary healthcare setting (Figure 3b). Patients remaining in NYHA class III–IV despite HFpEF treatment, and/or with complex management characteristics, including refractory congestion and/or frequent hospitalizations, severe renal disease (estimated glomerular filtration rate ≤30 ml/min and/or urine albumin–creatinine ratio ≥300 mg/g), severe electrolyte disturbances, significant coronary artery disease, refractory cardiac arrhythmias and/or indication for ablation/cardioversion, severe comorbidities, uncertain etiologies (e.g. amyloidosis) and/or LVEF ≥65%, should be referred to a hospital setting for advanced investigation and/or treatment.
An angiotensin receptor/neprilysin inhibitor (ARNi) may also be considered in women across the LVEF range, and in men with LVEF between 50 and 60%, or alternatively an angiotensin receptor blocker (ARB) or an angiotensin-converting enzyme inhibitor (ACEi), for patients who cannot take ARNis due to intolerance or financial barriers (Figure 3c).16,38
In both primary healthcare and hospital settings, comprehensive HFpEF treatment always includes management of comorbidities, as well as education in disease awareness and self-care for patients and informal caregivers, exercise, and vaccination promotion. Patients may be referred to a cardiac rehabilitation program if one is available. Referral for palliative care should be considered when deemed appropriate. Social needs should be assessed and addressed.
Patient-centered multidisciplinary care for heart failure with preserved ejection fraction in PortugalThe organization of HFpEF care should be patient-centered and should include multidisciplinary integrated care teams involving cardiologists, internists, primary care physicians, nurses, and other health professionals, linked together in a seamless care system. These teams should ultimately work together to reduce morbidity and mortality and to improve patients’ functional capacity and quality of life. The contribution of social workers and informal caregivers is essential. The creation of Local Health Units (Unidades Locais de Saúde) all over Portugal as an organizational model of care should help to disseminate this integrative and collaborative patient-centered approach to the country's HFpEF patients.
ConclusionsHFpEF is highly prevalent in Portugal, as shown by the PORTHOS study, particularly in older patients.
This represents an extraordinary challenge to organize a national system of HFpEF diagnosis and treatment. Primary care physicians are central to identifying these patients, initiating therapy and referring them to hospital when necessary. To do so, measurement of NPs is desirable and high-quality echocardiograms are crucial to providing the most accurate assessments. Additionally, a comprehensive approach, consisting of multidisciplinary teams and a systematic, pragmatic vision, are key to improving HFpEF management, helping to address all the patient's needs and providing patient-centered care.
In this paper, we propose a holistic roadmap intended to guide clinicians from all specialties in the pragmatic management of HFpEF patients throughout the disease trajectory, from diagnosis to treatment, and to promote appropriate coordination between primary and hospital care, built on the most recent international HF guidelines and on the position of a multidisciplinary and representative HF national expert panel.
FundingThe authors meet criteria for authorship as recommended by the ICMJE.
The authors did not receive payment related to the development of the manuscripts.
Ana Santos of Prime Focus provided medical writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim and Lilly Portugal.
Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Conflicts of interestJSC, EM, MO, MP, DB, RC, ALM, LG, MF, FF, HP, MS have no conflicts of interest to declare.
RTM is an employee of Boehringer Ingelheim.
PMS has received speaker/consultant fees from AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Novartis, and Novo Nordisk.
NC has received speaker fees and participated in congresses supported by Bristol Myers Squibb, Cytokinetics, Pfizer, Boehringer, Ferrer, and Bial.
CG has received speaker and/or advisory board fees from AstraZeneca, Bial, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Servier.
BM has received speaker or advisory fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Servier, Vifor Pharma, and Viartris.
IA has received speaker fees from AstraZeneca, Bial, Novartis, Servier, and advisory fees from AstraZeneca, Novartis, and Boehringer Ingelheim/Lilly.
PS has received speaker or consultancy fees, or investigational grants from AstraZeneca, Boehringer Ingelheim, Ferrer, GSK, Lilly, Medinfar, MSD, and Tecnimede.
CF has received speaker and consultant fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Roche, Sanofi, and CSL Vifor.
JPF has performed consultancy for Boehringer Ingelheim, Novartis, Bayer, Salamandra, Bial, AstraZeneca, and Abbott.
IM has received speaker and consultancy fees from AstraZeneca, Bayer, Bial, CSL Vifor, Daiichi-Sankyo, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Servier.
AA has received speaker or advisory board fees from AstraZeneca, Boehringer Ingelheim, and Bial.
RB has received consultancy and/or speaking fees from AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Lilly, MSD, Novartis, Servier and Vifor Pharma.
JS has received speaker/consultant fees from AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Roche, Merck, Viatris, and Pfizer.
JF has received speaker and consultant fees from Amgen, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis.
CL received speaker/consultant fees from Boehringer, Bial, and Novartis.
CA has received speaker and consultant fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, and Servier.
RFC has received speaker and consultant fees from AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Novartis, and Servier.
EM has received speaker and consultant fees from AstraZeneca, Bial, Amicus Therapeutics, Sanofi, Amgen, and Pfizer.
JP has received speaker/consultant fees from AstraZeneca, Bayer, Bial, Boehringer Ingelheim/Lilly, Daiichi-Sankyo, GSK, Novartis, Pfizer, Servier, and Vifor Pharma.
We would like to thank Ana Santos, medical writer at Prime Focus, for her excellence in scientific accuracy, consolidation of co-authors’ contributions, and data management for an extensive expert panel, over the two-year project execution period.