The therapeutic potential of exercise training in the mobilization of endothelial progenitor cells (EPCs) into the peripheral blood in patients with cardiovascular disease is not yet clear. A systematic review and meta-analysis was performed in order to assess the effectiveness of exercise training in increasing the number of circulating EPCs in patients with cardiovascular disease. A literature search was conducted across the PubMed, Scopus, Web of Science and EBSCO databases, including the reference lists of relevant papers. The quality of randomized clinical trials was evaluated using the PEDro scale. The primary outcome data were circulating EPC levels. Six studies (236 participants) – three on heart failure (n=111), one on peripheral arterial disease (n=40) and two on coronary artery disease (n=85) – were included. There was an increase in EPC levels in the exercise training groups (effect size [ES]=0.57; 95% CI: 0.01-1.12), with considerable heterogeneity (I2=75.6%; p<0.001). In subgroup analyses, patients with heart failure showed a significant increase in EPCs in the exercise training groups (ES=0.52; 95% CI: 0.15-0.90), with low heterogeneity (I2=0.0%; p=0.648), while no significant increase (ES=0.67; 95% CI: -0.70-2.04; I2=91.2%; p<0.001) was observed in patients with arterial disease. The only study in patients with peripheral arterial disease showed a significant increase in EPC levels. This meta-analysis indicates that exercise training may be a therapeutic option to improve EPC levels and potentially to enhance endothelial function and repair in patients with heart failure.
O potencial terapêutico do exercício físico na mobilização das células progenitoras endoteliais para circulação em doentes com doença cardiovascular não está ainda completamente estabelecido. Foi realizada uma revisão sistemática e meta-análise com o objetivo de determinar a eficácia do exercício físico no aumento do número circulante de células progenitoras endoteliais em doentes com doença cardiovascular. Foi realizada uma pesquisa nas bases de dados PubMed, Scopus, Web of Science e EBSCO, incluindo a lista de referências de artigos relevantes. A qualidade dos estudos foi avaliada pela escala PEDro. Foram incluídos seis estudos (236 participantes), três em doentes com insuficiência cardíaca (n=111), um com doença arterial periférica (n=40) e dois em doença das artérias coronárias (n=85). O exercício físico melhorou o número de células progenitoras endoteliais em doentes com doença cardiovascular (ES=0,57; IC95%: 0,01; 1,12), com considerável heterogeneidade (I2=75,6%; p<0,001). Na análise de subgrupo, os doentes com insuficiência cardíaca apresentaram um aumento significativo nas células progenitoras endoteliais em favor do grupo de exercício (ES=0,52; IC95%: 0,15; 0,90), com baixo risco de heterogeneidade (I2=0,0%; p=0,648), não se observando aumento significativo (ES=0,67; IC95%: -0,70; 2,04; I2=91,2%; p<0,001) nos doentes com doença arterial. O único estudo em doentes com doença arterial periférica reportou um aumento significativo nas células progenitoras endoteliais. Esta meta-análise indica que o exercício físico pode ser uma opção terapêutica para melhorar os níveis das células progenitoras endoteliais e, potencialmente, melhorar a função e regeneração endotelial, em doentes com insuficiência cardíaca.
Cardiovascular disease (CVD) is the leading cause of death worldwide.1 In order to decrease the high mortality burden attributable to CVD, many clinical approaches have focused on endothelial health, as a therapeutic target and an indicator of prognosis.2–4 The endothelium has essential functions in cardiovascular health, including regulating the balance of thrombotic and anticoagulant factors, regulating vascular tone and blood flow, self-repair, and promotion of angiogenesis and blood perfusion.5–7 Conversely, dysfunctional and/or damaged endothelium increases the risk for progression of atherosclerosis,8 and is considered an underlying cause in the development of CVD and an independent predictor of cardiovascular events.6,9 Endothelial health is modulated by various behavioral factors such as smoking,10 diet11 and physical exercise.12 Exercise training is a class IA intervention in patients with stable angina, previous myocardial infarction, and coronary artery bypass graft surgery or percutaneous coronary intervention,13 and in patients with heart failure (HF).14 Among other benefits, exercise training improves cardiorespiratory fitness and quality of life,15,16 and mitigates progression of atherosclerosis by enhancing vascular homeostasis17 and endothelial function.12,18
The number of endothelial progenitor cells (EPCs) is positively correlated with vascular function and inversely correlated with the Framingham risk score,19 and predicts morbidity and mortality in patients with CVD.20,21 EPCs are mobilized to sites of endothelial injury and differentiate into mature endothelial cells, repairing and maintaining vascular structure, in addition to mediating paracrine mechanisms and promoting angiogenesis. The role of EPCs in vascular health is thus crucial to the treatment of CVD.22
Although some previous studies have shown that exercise training induces the mobilization of EPCs from the bone marrow to the peripheral blood,23–26 others have not demonstrated this effect.27,28 This topic has been extensively reviewed; several narrative reviews29–45 and systematic reviews12,24,46 can be found in the literature summarizing the effects and potential mechanisms by which exercise mobilizes EPCs. Nevertheless, only Pearson and Smart12 have performed a meta-analysis, which also included non-randomized controlled trials (RCTs). Additionally, analysis of the impact of exercise on EPC numbers was a secondary aim of their analysis,12 which may have limited further interpretations on this issue, and was restricted to studies enrolling patients with HF. In view of the above, the aim of this study is to conduct a systematic review and meta-analysis of RCTs investigating the effects of physical exercise on the circulating number and function of EPCs in patients with CVD.
Databases and search strategyThis systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.47 Relevant studies were identified by searching the PubMed, Scopus, Web of Science and EBSCO databases. The search terms were the following: “cardiovascular disease*” OR “chronic disease*” OR “heart disease*” OR hypertension OR “peripheral arterial disease” OR diabetes mellitus OR “coronary artery disease” OR “heart failure” OR myocardial infarction OR “vascular disease*” in combination with “endothelial progenitor cells” OR “progenitor cells” OR “colony-forming units” OR “stem cells” OR “circulating angiogenic cells” in combination with “exercise training” OR exercise OR “cardiac rehabilitation” OR “physical exercise” OR “physical activity” OR “exercise therapy” OR “rehabilitation exercise” and avoiding the terms infant* OR child* OR rat* OR mice OR mouse OR animal* OR cancer OR neoplasm* OR neoplasia. All the keywords were MeSH terms, with the exception of “acute myocardial infarction”, “progenitor cells” and “circulating angiogenic cells”. Considering that EPCs were first isolated by Asahara et al. in 1997,48 we limited the search to studies published between January 1997 and January 31, 2018. In addition, we manually searched the reference lists of landmark studies and review articles on this topic to identify any relevant studies that electronic searches might have missed.
Study selectionStudies were eligible if they (i) were RCTs; (ii) included adults (≥18 years old) with established CVD; (iii) compared an experimental group receiving a structured program of exercise training, either exclusively or as a component of a cardiac rehabilitation program; (iv) included a control group receiving only standard medical care (that did not perform exercise training); (v) included an exercise training program lasting at least one week; (vi) assessed circulating EPC levels before and after the intervention; and (vii) were written in English. We excluded literature review papers, letters to the editor, abstracts published in conference proceedings, studies that assessed the acute effects of a single exercise session, studies involving healthy subjects or patients with other clinical conditions (e.g. kidney disease), and animal model studies.
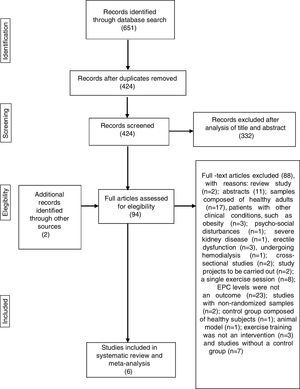
Initially a single author screened the titles and discarded duplicates. Subsequently, two authors independently determined whether studies fulfilled the criteria for inclusion in this review through examination of screened titles and abstracts. The same two authors then analyzed the full articles and used a standardized form to determine eligibility for inclusion in the review. Any disagreements regarding the inclusion of studies were resolved by discussion with a third author. The process of identification and selection of the studies is displayed in Figure 1.
Data extraction and assessment of methodological qualityData were extracted for the main outcome (circulating EPC levels) as well as for the study design, population, details of the intervention (frequency, mode, duration, intensity and characteristics of the exercise) and control groups, antibodies used to identify EPCs, units of EPC measurement, functionality of EPCs in vitro (proliferative capacity, migratory capacity, number of colony forming unit endothelial cells), time of blood sample collection for EPC analysis, and exercise capacity. The methodological quality of the studies was determined using the Physiotherapy Evidence Database (PEDro) scale. This scale comprises 10 items related to internal validity and interpretability; a higher score indicates better methodological quality.49 Based on the PEDro score, the quality of the included RCTs were described as following: 6-10 for high-quality studies; 4-5 for fair-quality studies; and ≤3 for poor-quality studies.49 Two authors independently extracted the above-mentioned data using a customized form and evaluated methodological quality, while a third author resolved disagreements.
Statistical analysisThe data were analyzed using STATA/SE software, version 14 (StataCorp, College Station, TX, USA). The standardized mean difference between the pre-post of the intervention group versus the control group was calculated for EPC levels using Cohen's d as the effect size statistic. The significance of the pooled effect size was estimated based on 95% confidence intervals (CI). Data not provided in the main text or tables were obtained by contacting the authors or (when there was no response) extracted from figures. One study50 included two intervention groups (a younger group ≤55 years old and an older group ≥65 years old); the data from these groups were analyzed as independent samples.
The heterogeneity of results across studies was quantified by I2 statistics as follows: low risk of heterogeneity (0-40%); moderate risk of heterogeneity (30-60%); substantial heterogeneity (50-90%); or considerable heterogeneity (75-100%). Significance levels were expressed as p-values.51
Subgroup analyses were performed based on the clinical condition: HF or arterial disease (peripheral and coronary).
Random-effects meta-regression was used to assess whether results differed according to the mean age of participants and the length of the intervention, since these could be considered sources of heterogeneity.
Publication bias was identified by visual inspection of funnel plots and the method proposed by Egger.52
Systematic reviewThe initial search identified 651 titles. After exclusion of 227 duplicates, the titles and abstracts of the remaining 424 articles were analyzed, following which 332 articles were excluded, resulting in 92 studies qualifying for full-paper assessment. Of the 92 potentially eligible articles, only four25,50,53,54 met the inclusion criteria (Figure 1). After screening the reference lists of relevant articles on this topic, two additional papers, not retrieved from the database search, were found.55,56 A total of six papers were therefore included in the systematic review and meta-analysis.
These six studies included 236 participants, 119 in exercise training and 117 in control groups (Table 1). The sample size ranged from 21 to 64 participants and overall the studies involved more men (n=189, almost 80%) than women (n=47). Two studies54,56 included coronary artery disease (CAD) patients (n=85), three studies50,53,55 involved patients with HF with reduced ejection fraction (n=111), and one study included patients with peripheral arterial disease (PAD) (n=40) with mild to severe claudication.25 All studies identified and quantified circulating EPCs by flow cytometry using a combination of specific antibodies: three studies used the specific antibodies CD34+/KDR+,50,54,56 two CD45dim/CD34+/KDR+,53,55 one CD34+/CD133+/KDR+25 and one CD133+/KDR+50. Three studies quantified EPCs as the percentage of mononuclear cells,25,53,55 two as the absolute number per ml of peripheral blood,25,50 one as the percentage of absolute cell counts divided by lymphocyte counts,56 and one as cells per 100 000 laser detection events.54 Blood collection for EPC determination was performed 24-48 hours,54 72 hours,25 and up to a week53 after the last exercise session. Some studies did not provide the time of blood collection.50,55,56 Only one study reported the time of day of blood collection, between 8:00 and 9:00 am.53
Main features of the included studies.
| Study | Sample | Exercise training characteristics | EPC determination | Main results (number and functionality of EPCs) | PEDro score |
|---|---|---|---|---|---|
| Schlager et al.25 | 40 PAD patients with intermittent claudicationEG: 20 (69±8 years; 13 men/7 women)CG: 20 (70±1 years; 11 men/9 women) | Mode: aerobicLength: 24 wksFrequency: 2 sessions/wkIntensity: walking speed that elicited claudication symptoms within 3-5 minSession: 50 min | CD34+/CD133+/KDR+ (% MNCs and absolute number/ml peripheral blood) | Number: ↑ percentage of EPCsFunctionality: ↑ migratory capacity;↑ CFU-EC (well) | 7/10 |
| Luk et al.56 | 64 stable CAD patientsEG: 32 (67.7±9 years; 24 men/8 women)CG: 32 (66.6±7 years; 24 men/8 women) | Mode: aerobic and resistance;Length: 8 wksFrequency: 3 sessions/wkIntensity: 80% HRmax (aerobic exercise) and perceived exertion and % HRmax (resistance exercise)Session: 60 min | CD34+/KDR+ (% absolute cell counts divided by lymphocyte counts) | Number: no significant changeFunctionality: not assessed | 6/10 |
| Eleuteri et al.53 | 21 male patients with HFEG: 11 (66±2 years)CG: 10 (63±2 years) | Mode: aerobic;Length: 12 wksFrequency: 5 sessions/wkIntensity: HR at ventilatory thresholdSession: 40 min | CD45dim/CD34+/KDR+ (% MNCs) | Number: ↑ percentage of EPCsFunctionality: not assessed | 5/10 |
| Mezzani et al.55 | 30 male patients with HFEG: 15 (65±7 years)CG: 15 (63±7 years) | Mode: aerobicLength: 12 wksFrequency: 5 sessions/wkIntensity: HR at ventilatory thresholdSession: 40 min | CD45dim/CD34+/KDR+ (% MNCs) | Number: ↑ percentage of EPCsFunctionality: not assessed | 6/10 |
| Gagliardi et al.54 | 21 stable CAD patientsEG: 11 (59.5±2 years; 7 men/4 women)CG: 10 (65.4±1.6 years; all men) | Mode: aerobicLength: 12 wksFrequency: 3 sessions/wkIntensity: not availableSession: not available | CD34+/KDR+ (cells/100 000 laser detection events) | Number: no significant changeFunctionality: not assessed | 6/10 |
| Sandri et al.50 | 69 HF patientsGroup 1(aged ≤55):EG: 15 (50±5 years; 12 men/3 women), CG: 30 (49±5 years; 13 men/2 women)Group 2 (aged ≥65):EG: 15 (72±4 years; 12 men/3 women), CG: 15 (72±3 years; 12 men/3 women) | Mode: aerobicLength: 4 wksFrequency: 5 sessions/wkIntensity: 70% VO2maxSession: 30 min (4 sessions) plus 60 min (1 session) | CD34+/KDR+ and CD133+/KDR+ (cells/ml) | Number: ↑ EPCs in both EGsFunctionality: ↑ migratory capacity in both EGs | 5/10 |
↑: increased; CAD: coronary artery disease; CFU-EC: colony forming unit endothelial cells; CG: control group; EG: experimental group; HF: heart failure; HR: heart rate; HRmax: maximum heart rate; MNCs: mononuclear cells; PAD: peripheral arterial disease; VO2max: maximum oxygen uptake; wk: week.
Regarding the characteristics of exercise training, all studies used aerobic exercise, either exclusively25,50,53–55 or combined with resistance exercise.56 The length of exercise interventions ranged from four50 to 24 weeks,25 with a frequency of two,25 three,54,56 or five50,53,55 sessions per week. The duration of the exercise sessions ranged from 30 to 60 min. Exercise intensity was determined using claudication symptoms,25 or heart rate at the ventilatory anaerobic threshold,53,55 at 80% of maximum heart rate (HRmax)56 or at 70% of maximum oxygen uptake.50 Only one study involved resistance exercise determined by intensity assessed through perceived exertion and HRmax, at a similar intensity to aerobic exercise (80% of HRmax).56 One study did not report either exercise session duration or exercise intensity.54
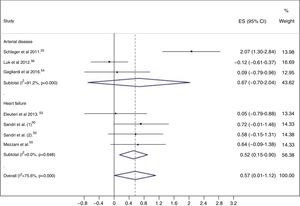
Meta-analysisWhen exercise training was compared to control, EPC levels were higher in the exercise training groups (ES=0.57; 95% CI: 0.01, 1.12), with considerable heterogeneity (I2=75.6%; p<0.001) (Figure 2).
Subgroup analyses and meta-regressionSubgroup analyses were performed based on the clinical condition, i.e. patients with HF and patients with arterial disease (CAD or PAD). Studies enrolling patients with CAD and PAD were grouped together, because only one study in patients with PAD was included in this review. In patients with HF, a significant increase in circulating EPC levels was seen in the exercise training group (ES=0.52; 95% CI: 0.15-0.90), with low heterogeneity (I2=0.0%; p=0.648) (Figure 2).
In patients with arterial disease, there was no significant increase in EPC levels in the exercise training groups (ES=0.67; 95% CI: -0.70-2.04), with considerable heterogeneity (I2=91.2%; p<0.001) (Figure 2). The two studies54,56 that enrolled patients with CAD showed no changes in circulating EPC numbers after the exercise training program, while the study25 that assessed the effects of aerobic exercise in the mobilization of EPCs in patients with PAD and intermittent claudication showed a significant increase in circulating EPC levels.
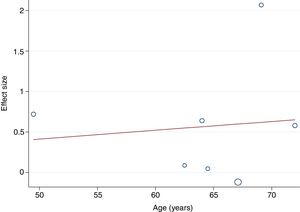
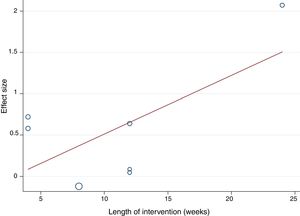
The random-effects meta-regression model showed that age (Figure 3) and length of the intervention (Figure 4) are not related to heterogeneity across studies (p=0.821 for age, p=0.113 for length of the intervention).
Sensitivity analysisThe pooled effect size estimate was not significantly altered in magnitude or direction when individual study data were removed from the analysis one at a time.
Study quality and publication biasThe studies had a mean score of 6 on the PEDro scale, ranging from 5 to 7. Two studies scored 5, which indicates fair methodological quality; the remaining studies were classified as high methodological quality RCTs (Table 1).
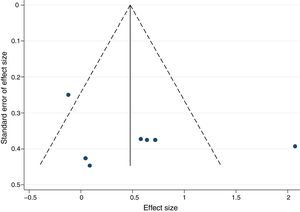
There was no significant publication bias for exercise training intervention versus control, as demonstrated by the asymmetry of the funnel plot (Figure 5) and Egger's test (p=0.324).
DiscussionThe present systematic review and meta-analysis provides a synthesis of the evidence, which suggests that exercise training interventions are successful in the mobilization of EPCs from bone marrow to circulation in patients with HF. Furthermore, the only study in patients with PAD also showed a significant increase in circulating EPC levels.
Our pooled data demonstrated that exercise training had a significant effect on EPCs in patients with HF. This finding is consistent with previous studies in patients with HF, which show an increase in the circulating levels and in-vitro functionality of EPCs after an aerobic exercise program.57–59 A previous systematic review and meta-analysis quantifying the effect of exercise training on endothelial function in heart failure patients, assessed by flow-mediated dilation, but examining as a secondary aim the effects of exercise training on EPCs, also showed a significant improvement in EPCs as a result of exercise training.12
Various mechanisms may explain the beneficial effects of exercise training on EPC levels. In brief, exercise training influences the mobilization of EPCs by increasing shear stress and levels of nitric oxide and angiogenic factors, particularly vascular endothelial growth factor (VEGF), stromal cell-derived growth factor-1, hypoxia-inducible factor 1 (HIF-1) and matrix metallopeptidase-9.23,24,29 Ischemia is also a powerful stimulus for EPC mobilization, mainly due to the release of angiogenic factors such as VEGF and HIF-1.29,60,61 In this regard, the increase in EPCs observed in the study enrolling patients with PAD25 could be explained by exercise-induced transitory ischemia, since patients were trained until moderate claudication was elicited. Interestingly, a previous study showed that four weeks of aerobic exercise-induced transitory ischemia was associated with a 5.2-fold increase in CD34+/KDR+ cells in patients with PAD, whereas no changes were observed in the PAD and CAD groups who underwent non- exercise-induced ischemia.62
The absence of changes in EPC levels in patients with CAD in the RCTs included in this systematic review and meta-analysis has also been observed in other non-randomized trials.27,28 Nonetheless, conflicting responses to exercise training have been found in patients with CAD; some studies failed to show an increase in circulating EPC levels after continuous aerobic exercise at moderate intensity27,28 or interval training,27 while others showed an increase in EPC levels in response to an aerobic exercise program63,64 or combined exercise training.65 The lack of detailed descriptions regarding the dose of resistance training (number of series of exercise, number of repetitions, and resting time between series)56 and aerobic training (session duration and exercise intensity)54 in the included studies hinders comparisons between results and replication of their methodologies. The possibility that the exercise training protocol delivered a suboptimal shear stress stimulus that was insufficient to increase the mobilization of EPCs is noted as a possible explanation for the results observed in patients with CAD. Due to these limitations, these results should be interpreted with caution and future randomized trials are clearly needed in this population to enhance understanding of the effects of exercise training on EPCs in patients with CAD.
Study limitationsSome limitations of this review should be acknowledged. A major limitation is the considerable heterogeneity among studies. Differences in the methodological assessment of EPCs may have contributed to this level of heterogeneity. EPCs are a relatively scarce cell population, which makes their quantification by flow cytometry a challenging task.66 The best EPC phenotype to determine EPCs in a clinical setting appears to be CD34+/KDR+/CD45dim.66–68 The results of different studies may also to some extent be influenced by the lack of consistency in isolation strategies, use of lineage markers to determine EPCs, and units used to report EPCs.22 In this regard, the use of cells per ml as units to quantify EPCs50 is a major limitation, due to the hemodilution that can be seen in patients with HF. Also, several studies did not clearly state the time of day of blood collection and the time from the last exercise session, as EPCs appear to display diurnal variation69 and the timing of blood collection post-exercise could reflect the effect of the last exercise session.23,70 The differences between studies in disease severity, medication use and cardiovascular risk factors may also have contributed to the high heterogeneity. Data from some studies were extracted from figures, which is another potential source of error. The lack of a clear description of the characteristics (e.g., intensity, mode, duration, frequency) of the exercise training programs was also a limitation.
Future prospectsThis analysis has several implications for further research. In most studies, the sample was composed mainly (or exclusively) of male patients; therefore, future studies should include mixed-gender samples and sub-analysis by gender, when possible, to ascertain the possible existence of between-gender differences, since hormones could influence EPC levels.71 It would be useful to assess endothelial function and correlate any changes with changes in EPC levels; the only study included in this review that assessed the relation between endothelial function and EPCs found no correlation. Although the effect of exercise on both endothelial function and EPC levels shares common mechanisms (decreasing inflammatory cytokine concentrations and increasing endothelial nitric oxide synthase activity and endothelium-dependent nitric oxide production),12,29,72 the relation between the two variables still lacks clarification. Future studies should use standard gating strategies and assessment protocols for the quantification of EPCs.
ConclusionsThis meta-analysis found that exercise training improves the number of circulating EPCs in patients with HF with reduced ejection fraction. This increase in circulating EPC levels may enhance endothelial function and repair. However, whether the same effects occur in patients with preserved ejection fraction, or whether the positive results observed in the only study in patients with PAD are replicated in further studies, is still unclear.
Conflicts of interestThe authors have no conflicts of interest to declare.
The Research Centre in Physical Activity, Health and Leisure (CIAFEL) is funded by the European Regional Development Fund through the Operational Competitiveness Programme (COMPETE) and by the FCT (grant UIDB/00617/2020). IBiMED is a research unit supported by the Portuguese Foundation for Science and Technology (REF: UID/BIM/04501/2020) and FEDER/Compete2020 funds. This work was supported by FCT under the grant PTDC/MEC-CAR/30011/2017 and by the FEDER under the grant POCI-01-0145-FEDER-030011.