The increasing incidence of ischemic heart disease is a serious threat to human health. Increased CASC15, a long non-coding RNA, has been shown to adversely affect cardiac muscle. The objective of this paper was to explore the effect of CASC15 on a cell model of myocardial infarction and its possible mechanism.
MethodsH9c2 cells were selected to establish the myocardial infarction model through hypoxia/reoxygenation (H/R) treatment. The expression of CASC15 was attenuated by cell transfection in vitro. The level of CASC15 was detected by RT-qPCR. Cell viability was detected by CCK-8 assay, and cell apoptosis was assessed by flow cytometry. The content of MDA and the activity of SOD and GSH-Px were measured by ELISA. Luciferase reporter gene assay was used to determine the relationship between CASC15 and miRNA.
ResultsCASC15 expression was increased in H/R-treated H9c2 cells. Overexpression of CASC15 adversely affected cell viability and promoted H/R-induced oxidative stress. Inhibition of CASC15 promoted cell viability and suppressed cell apoptosis and oxidative stress damage. Additionally, luciferase reporter gene assay confirmed the targeting relationship between CASC15 and miR-542-3p, and attenuating CASC15 expression enhanced the level of miR-542-3p. Reduction of miR-542-3p weakened the viability of the H/R cell model, increased apoptosis, and enhanced oxidative stress damage.
ConclusionThis study suggests that overexpression of CASC15 may inhibit the viability of H9c2 cells, promote apoptosis and induce oxidative stress through targeted regulation of miR-542-3p expression.
O aumento da doença isquémica cardíaca representa uma séria ameaça à saúde humana. Um aumento no CASC15, um RNA não codificante longo, está associado à lesão do músculo cardíaco. O objetivo deste trabalho foi explorar o mecanismo celular através do qual a CASC15 contribui para o enfarte do miocárdio.
MétodosCélulas H9c2 expostas a hipóxia/reoxigenação (H/R) foram selecionadas como modelo para estudar os mecanismos envolvidos no enfarto do miocárdio. A expressão de CASC15 foi inibida pela transfecção com siRNA. Os níveis de CASC15 foram avaliados por RT-qPCR e a viabilidade celular e apoptose determinadas pelo ensaio CCK-8 e citometria de fluxo, respetivamente. Os teores de MDA, SOD e GSH-Px foram medidos por ELISA. O ensaio do gene repórter da luciferase foi utilizado para verificar a relação entre CASC15 e os níveis do miRNA miR-542-3p.
ResultadosCélulas H9c2 sujeitas a H/R apresentam níveis aumentados de CASC15. A sobre-expressão de CASC15 afetou negativamente a função celular e promoveu a lesão induzida pelo stress associado H/R. Por outro lado, a inibição do CASC15 promoveu viabilidade celular e suprimiu apoptose celular e os danos oxidativos. Além disso, o ensaio do gene repórter da luciferase confirmou a relação alvo entre CASC15 e miR-542-3p, com uma diminuição da expressão de CASC15 a resultar num aumento do nível de miR-542-3p. Uma diminuição do miR-542-3p comprometeu a viabilidade do modelo de células H/R e aumentou a apoptose e o dano oxidativo.
ConclusãoEste estudo sugere que um aumento da expressão de CASC15 durante a H/R pode contribuir para diminuir a viabilidade dos cardiomiócitos, promover apoptose e induzir stress oxidativo, através da regulação da expressão de miR-542-3p.
Acute myocardial infarction (AMI) and other types of cardiovascular disease are common entities. With the aging of populations, the incidence and mortality of cardiovascular disease have increased significantly.1 Therefore, exploring the molecular mechanisms underlying the occurrence and development of cardiovascular disease is of great significance for the prevention and treatment of these conditions. For ischemic heart disease such as AMI, restoring blood flow to ischemic myocardial tissue through revascularization is the most effective method to save life at present.2 However, studies have confirmed that such treatment can also cause myocardial ischemia–reperfusion (I/R) injury when coronary blood flow is restored, which significantly reduces the therapeutic effect.3 Therefore, how to reduce myocardial I/R injury has become a focus of research.
Long non-coding RNA (lncRNA) consists of endogenous non-coding RNA sequences that can regulate the expression of target genes by acting as microRNA sponges, and thus participate in many biological processes such as the development of diseases and tumors.4,5 Previous studies have reported that lncRNAs play a crucial role in myocardial I/R injury. For example, a study by Long et al. showed that the expression of lncRNA FTX decreased in injured myocardium and inhibited the apoptosis of cardiomyocytes by targeting miR-29b-1-5p.6 The mechanism of lncRNAs in myocardial I/R injury remains unknown. Cancer susceptibility 15 (CASC15), an lncRNA on human chromosome 6p22.3, has been shown to play a regulatory role in the growth, metastasis and drug resistance of breast cancer, colorectal cancer, hepatocellular carcinoma, melanoma and other malignant tumors.7–10 Yu et al. found that CASC15 promoted cell proliferation and metastasis in non-small cell lung carcinoma by sponge adsorption of miR-130b-3p in vitro and in vivo.11 In recent years, its expression has been found to be abnormal in cardiovascular disease. A study showed that compared to healthy volunteers, serum CASC15 was increased in patients with atherosclerosis and AMI.12 However, the mechanism of CASC15 in I/R injury has not been fully elucidated.
ObjectivesIn this study, an AMI cell model was constructed by hypoxia/reoxygenation (H/R) treatment, and the mechanism of CASC15 in I/R injury was explored through this model, which provided a theoretical basis for the prevention and treatment of I/R injury in patients with AMI.
MethodsCell culture and treatmentH9c2 cells were purchased from the American Type Culture Collection and were grown in Dulbecco's Modified Eagle Medium (DMEM) (containing 10% fetal bovine serum [FBS] and 1% penicillin–streptomycin mixture) in an incubator containing 5% CO2 at 37°C.
The AMI cell model was constructed by inducing H/R. Briefly, H9c2 cells were cultured in serum-free DMEM and then placed in a hypoxic incubator containing 5% CO2 and 95% N2 for 30 min. The FBS-free DMEM was then replaced with DMEM containing 10% FBS, and the cells were placed in a conventional incubator containing 5% CO2 and 95% air for reoxygenation. Cells cultured under conventional conditions were used as a control group. After 24 h of reoxygenation, cells were collected for subsequent experimental operations.
Cell transfectionSmall interfering RNA (siRNA) against CASC15 (si-CASC15), si-negative control (si-NC), miR-542-3p mimic, miR-542-3p inhibitor, mimic NC and inhibitor negative control (NC) were purchased from GenePharma (Shanghai, China). H9c2 cells were inoculated in 24-well plates and cultured overnight. The above oligonucleotides were mixed with the transfection reagent Lipofectamine 2000 for 10 min at room temperature and then diluted with serum-free DMEM to a final concentration of 50 nM. Cell transfection procedures were performed according to the instructions for Lipofectamine 2000. After transfection for 24 h, cells were subjected to H/R treatment.
Reverse transcription-quantitative polymerase chain reactionRNA was extracted using TRIzol reagent. The PrimeScript RT kit was used for cDNA synthesis, and the SYBR Green PCR kit was used for the polymerase chain reaction (PCR) reaction. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 were used as an internal reference for CASC15 and U6, respectively. The primers used were as follows: CASC15: forward: 5′-CTTTGTCTGCTCCGGGACT-3′, reverse: 5′-TTAAGGGACATTTCCCCCG-3′; miR-542-3p: forward: 5′-TCGGGGATCATCATGTCA-3′, reverse: 5′-GAGTGGCTCCCAGACCTT-3′; GAPDH: forward: 5′-GTGGCTCCCAGACCTTTC-3′, reverse: 5′-AGCTTGACAAAGTGGTCG-3′; U6: forward: 5′-CTCGCTTCGGCAGCACA-3′, reverse: 5′-AACGCTTCACGAATTTGCGT-3′. Reaction conditions were initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 92°C for 15 s, annealing at 60°C for 1 min and extension at 60°C for 1 min.
Cell viability assayCell viability was assessed with the Cell Counting Kit-8 assay (CCK-8). H9c2 cells were seeded in 96-well plates at a density of 4000 cells/well and cultured overnight. After cell transfection and H/R treatment according to the experimental procedure, cell viability was monitored every 24 h according to the manufacturer's scheme, whereby CCK-8 solution at a concentration of 10 nM was added to the cells at a preset time point (0 h, 24 h, 48 h, 72 h, 96 h), and the cells were incubated in the dark at 37°C for 2 h, and then optical density values were measured at 450 nm.
Cell apoptosis assayCell apoptosis was detected by flow cytometry. H9c2 cells were seeded in 6-well plates and treated according to the steps of transfection and H/R treatment. They were then harvested and stained with the Annexin V-FITC/PI apoptosis detection kit. After staining, the cell apoptosis rate was immediately determined by flow cytometry.
Assessment of oxidative stress indexCell supernatants were used to determine superoxide dismutase (SOD), methylenedioxyamphetamine (MDA), and glutathione peroxidase (GSH-Px) content. The corresponding kits were purchased from Nanjing Jiancheng Biotechnology Co., Ltd., as follows: SOD assay kit (Sigma-Aldrich, cat. CS1000) using the water-soluble tetrazolium method; MDA assay kit (Beyotime, cat. S0131) using the thiobarbituric acid method; and GSH-Px assay kit (Nanjing Jiancheng Bioengineering Institute, cat. A005) using the colorimetric method. Changes in absorbance were detected by spectrophotometry.
Luciferase reporter assayStarbase v2.0 was used to analyze RNA–RNA interactions, and predicted that miR-542-3p had a complementary binding site to CASC15. We hypothesized that these two molecules have a targeted binding relationship, and tested this hypothesis by luciferase reporter gene assay. Briefly, the steps are as follows: wild-type CASC15 (WT-CASC15) and mutant CASC15 (mut-CASC15) were inserted into pmirGLO reporter vectors, and these reporter vectors were co-transfected into H9c2 cells with miR-542-3p mimic or miR-542-3p inhibitor using Lipofectamine 2000. After transfection for 48 h, relative luciferase activity was measured on a dual-luciferase reporter assay system. Renilla was used as an internal reference, and data were expressed as the ratio of Renilla luciferase activity to Firefly luciferase activity.
Statistical analysisData are presented as mean±standard deviation. The two-tailed Student's t test was used to compare the two groups, and multiple groups were compared by one-way analysis of variance. Statistical significance was assumed for p<0.05. Each test was repeated in triplicate.
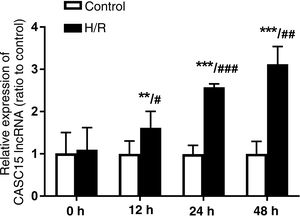
ResultsDetermination of reoxygenation timeReoxygenation times are presented in Figure 1. They show that CASC15 expression of cells in the H/R group was significantly higher than in the control group after reoxygenation (except at 0 h) (p<0.05). In addition, with the time extension, after reoxygenation culture, there were differences between the groups. CASC15 levels in the H/R group at 24 h were significantly higher than at 12 h (p<0.001), and similarly were significantly higher at 48 h than at 24 h (p<0.01). Therefore, 24 h was selected as the reoxygenation culture time for this study.
Effect of CASC15 downregulation on cell survivalThe intracellular CASC15 expression level was regulated by cell transfection. As shown in Figure 2A, CASC15 expression in the H/R model group was significantly increased compared with the control group, however, si-CASC15 transfection significantly downregulated CASC15 levels (p<0.001). Investigation of cell viability showed that compared with the control group, cell viability in the H/R model group was significantly inhibited, but was significantly promoted after CASC15 was inhibited (p<0.001) (Figure 2B). Concerning cell apoptosis, the proportion of apoptotic cells in the H/R model group was about 20%, which was significantly higher than in the control group. As expected, CASC15 depletion helped alleviate H/R-induced apoptosis (p<0.001) (Figure 2C).
Validation of cell transfection, viability, and apoptosis. (A) Transfection of si-CASC15 effectively inhibited CASC15 expression in the H/R group; (B) inhibition of CASC15 promoted cell viability in the H/R group; (C) loss of CASC15 alleviated cell apoptosis in the H/R group. ***p<0.001 vs. control group; ###p<0.001 vs. Si-NC group. H/R: hypoxia/reoxygenation; lncRNA: long non-coding RNA; OD: optical density; si-CASC15: small interfering RNA against CASC15; si-NC: small interfering RNA as negative control.
It is well known that H/R induces oxidative damage. In the assessment of oxidative stress indicators, it was found that MDA levels in the H/R model group increased significantly, while SOD and GSH-Px levels decreased, indicating that H/R treatment caused oxidative damage to H9c2 cells (p<0.001) (Figure 3A–C). It is worth noting that after CASC15 expression was suppressed, oxidative damage was significantly reduced, mainly manifested by decreased MDA and increased SOD and GSH-Px (p<0.001) (Figure 3A–C).
Measurement of oxidative stress index. Silencing CASC15 alleviated H/R-induced oxidative stress injury, as shown by decreased production of (A) MDA and increased activity of (B) SOD and (C) GSH-Px. ***p<0.001 vs. control group; ###p<0.001 vs. Si-NC group. GSH-Px: glutathione peroxidase; H/R: hypoxia/reoxygenation; MDA: methylenedioxyamphetamine; si-CASC15: small interfering RNA against CASC15; si-NC: small interfering RNA as negative control; SOD: superoxide dismutase.
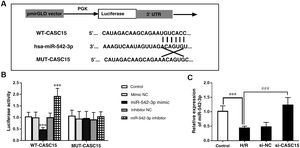
Bioinformatics predicted that CASC15 and miR-542-3p may have a targeting relationship, and the complementary binding sites of the two molecules are shown in Figure 4A. The luciferase reporter gene was used to verify this relationship, and the results showed that for the WT-CASC15 group, transfection of miR-542-3p mimic or inhibitor respectively weakened or enhanced luciferase activity, while no similar results were obtained in the mut-CASC15 group (Figure 4B) (p<0.001). Further verification results were as follows: in H/R model cells, miR-542-3p expression decreased, and at the same time, inhibition of CASC15 helped to improve the expression of miR-542-3p, which further underlines the relationship between CASC15 and miR-542-3p (p<0.001) (Figure 4C).
Verification of the relationship between CASC15 and miR-542-3p. (A) Binding site of CASC15 and miR-542-3p; (B) luciferase reporter gene assay; (C) miR-542-3p expression increased after inhibition of CASC15 in the H/R group. ***p<0.001 vs. control group; ###p<0.001 vs. Si-NC group. H/R: hypoxia/reoxygenation; mut-CASC15: mutant CASC15; NC: negative control; si-CASC15: small interfering RNA against CASC15; si-NC: small interfering RNA as negative control; WT-CASC15: wild-type CASC15.
As shown in Figure 5A, miR-542-3p expression, which increased due to the restricted expression of CASC15, was significantly downregulated after transfection with miR-542-3p inhibitor (p<0.001). The cell viability assay showed that inhibition of miR-542-3p reversed the increase in cell viability resulting from silencing CASC15 (p<0.001) (Figure 5B). Similarly, assessment of cell apoptosis showed that the loss of miR-542-3p promoted cell apoptosis compared to the inhibition of apoptosis induced by the attenuation of CASC15 (p<0.001) (Figure 5C).
Effect of miR-542-3p on cell viability and apoptosis. (A) Transfection with miR-542-3p inhibitor counteracted the enhanced effect of CASC15 knockdown on miR-542-3p expression; (B) downregulation of miR-542-3p reduced cell viability enhancement caused by CASC15 silencing; (C) loss of miR-542-3p increased the number of apoptotic cells in the si-CASC15 group. ###p<0.001 vs. Si-NC group; &&&p<0.001 vs. Si-CASC15 group. NC: negative control; OD: optical density; si-CASC15: small interfering RNA against CASC15; si-NC: small interfering RNA as negative control.
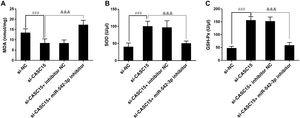
As mentioned above, H/R-induced oxidative stress damage was alleviated by the inhibition of CASC15. However, oxidative stress damage was found to be worsened when miR-542-3p in the cells was knocked down. It was observed that after miR-542-3p was inhibited, MDA levels increased and SOD and GSH-Px levels decreased (p<0.001) (Figure 6A–C).
Effect of miR-542-3p on oxidative stress injury. Downregulation of CASC15 expression had an inhibitory effect on oxidative stress injury in the H/R group, and this inhibitory effect was abolished by the deletion of miR-542-3p. With the absence of miR-542-3p, (A) MDA concentration and (B) SOD and (C) GSH-Px contents increased. ###p<0.001 vs. Si-NC group; &&&p<0.001 vs. Si-CASC15 group. GSH-Px: glutathione peroxidase; MDA: methylenedioxyamphetamine; si-CASC15: small interfering RNA against CASC15; si-NC: small interfering RNA as negative control; SOD: superoxide dismutase.
Abnormal lncRNA expression following I/R after AMI may be involved in the occurrence and development of myocardial injury. However, the regulatory mechanism of some lncRNAs in I/R injury remains unclear. The oncogenic effects of CASC15 have been demonstrated by its upregulation in liver cancer and promotion of hepatic carcinoma.13 Recent reports indicate that CASC15 is upregulated in acute ischemic stroke, and can function as a sponge molecule of miR-338-3p, promoting inflammatory damage to nerve cells.14 The results of our study showed that CASC15 expression in cardiomyocytes treated with H/R was significantly increased compared with normal cultured cardiomyocytes, suggesting that the abnormal increase in CASC15 was closely related to the occurrence of H/R injury. Further experiments showed that CASC15 inhibition significantly improved the H/R-induced dysfunction of H9c2 cells (including inhibition of cell viability and enhancement of cell apoptosis) and oxidative stress response. In addition, dual-luciferase reporter assay revealed that CASC15 targets miR-542-3p and that CASC15 expression was negatively regulated by miR-542-3p, indicating that CASC15 may promote H/R injury in H9c2 cells by suppressing the expression of miR-542-3p.
I/R injury is a common perioperative complication, especially in the reoxygenation phase, in which oxidative stress and a large increase in reactive oxygen species activate the mitochondrial apoptotic program, triggering the caspase cascade and ultimately leading to cardiomyocyte apoptosis,15,16 a key factor in the development of heart failure after I/R injury. It is therefore important to inhibit cardiomyocyte apoptosis for prevention and treatment of the consequences of I/R injury.17 In the present study, cell viability was significantly inhibited after H/R treatment, and the proportion of apoptotic cells was significantly greater than in the control group. Surprisingly, after CASC15 knockout and H/R treatment, the effect on cell viability and apoptosis was no longer serious, implying that excessive CASC15 plays an important role in H/R injury. Induction of apoptosis by CASC15 has been demonstrated in several studies. For example, Qin et al. showed that CASC15 expression increased in diabetes-induced chronic renal failure, and overexpression of CASC15 promoted podocyte apoptosis by sponging miR-34c.18 The molecular mechanism by which CASC15 promotes apoptosis of cardiomyocytes in our study needs further investigation.
Under normal physiological conditions, there is a dynamic balance between oxidation and antioxidation in the body. In the process of I/R damage, excessive production of oxidants leads to an imbalance between the oxidant and antioxidant systems.19 This imbalance results in oxidative stress, which leads not only to inflammation, but also to apoptosis, which is a major factor in aging and disease.20 Presently, the levels of three substances are commonly used as indices to assess the degree of oxidative stress: MDA, SOD and GSH-Px. MDA is an end-product of lipid peroxidation, and when increased indicates oxidative damage to the cell membrane.21 SOD activity is closely associated with oxidative damage caused by free radicals, and may indirectly reflect the body's ability to scavenge free radicals and prevent peroxidation of low-density lipoprotein.22 GSH-Px catalyzes the formation of glutathione disulfide (GSSG) from reduced monomeric glutathione (GSH), and its activity can be used as a biochemical index to measure antioxidant levels in cells.23 In the current study, after H/R treatment, oxidative stress indicators such as MDA increased while SOD and GSH-Px decreased, indicating that H9c2 cells were damaged by oxidation. However, after downregulation of CASC15, MDA concentrations in cells decreased, while SOD and GSH-Px levels increased, indicating that reduction of CASC15 alleviates the oxidative damage in cells caused by H/R and significantly enhances the antioxidant capacity of cells.
miRNAs can inhibit the expression of target genes by recognizing the corresponding target site in the 3’-UTR region of the target gene and binding to it through complementary base pairing.24 Studies have shown that miRNAs can influence the process of I/R injury by inhibiting oxidative damage and apoptosis of cardiomyocytes. As a tumor inhibitor, miR-542-3p mRNA expression is abnormally downregulated in many human tumors and other diseases, including colon cancer,25 prostate cancer,26 gastric cancer,27 and ischemic stroke.28 A previous study showed that miR-542-3p was significantly reduced in mice with middle cerebral artery occlusion, and overexpression of miR-542-3p mitigated apoptosis and activation of inflammatory responses caused by oxygen and glucose deprivation.29 Another study confirmed that overexpression of miR-542-3p has a protective effect on H/R-induced cardiomyocytes and reduces cell apoptosis.30 After a literature search, we selected miR-542-3p, which is clearly associated with cardiovascular disease, as the target of CASC15 in this study. Our results showed that the expression of miR-542-3p in cells was reduced after H/R treatment, suggesting that the loss of miR-542-3p may be involved in I/R injury. Furthermore, luciferase reporter assay confirmed that CASC15 targeted miR-542-3p, and that the latter can negatively regulate the expression of CASC15. In this study, H9c2 cells were co-transfected with si-CASC15 and miR-542-3p inhibitor, and then treated with H/R for 24 h. The results showed that CASC15 inhibition promoted H/R-induced cell viability, inhibited cell apoptosis and attenuated oxidative damage. However, the reduction of miR-542-3p significantly inhibited cardiomyocyte viability and promoted oxidative damage, which suggests that CASC15 overexpression may promote H/R-induced myocardial injury by inhibiting the expression of miR-542-3p.
ConclusionIn conclusion, CASC15 expression increased in H/R-induced H9c2 cells. Overexpression of CASC15 aggravated oxidative stress injury in H/R-induced cells, inhibited cardiomyocyte viability, and promoted cardiomyocyte apoptosis. CASC15 can target miR-542-3p. miR-542-3p expression decreased in the H/R group, which aggravated H/R-induced oxidative stress, inhibited cell viability and promoted apoptosis. Therefore, based on the above results, we preliminarily speculate that CASC15 may promote H/R-induced myocardial injury by inhibiting the expression of miR-542-3p in H/R-induced H9c2 cells.
FundingNo funding was received for conducting this study.
Conflicts of interestThe authors have no conflicts of interest to declare.