Hypertrophic cardiomyopathy (HCM) is accompanied by pathophysiological changes that predispose to the development of atrial fibrillation (AF). This arrhythmia impacts negatively on the morbidity, mortality and quality of life of these patients. Our objective was to evaluate the behavior of left atrial function, by means of atrial strain (derived from speckle tracking) and volumetric analysis by three-dimensional echocardiography, in patients with HCM with paroxysmal AF.
MethodWe analysed left atrial function in 53 patients with HCM, 25 of whom were paroxysmal AF carriers (mean age 61.7±9.9 years; 56% female) compared with 28 members of the control group (mean age 60.5±10 years; 53.6% female) who were matched especially for sex, age and other demographic data.
ResultsIt was observed that patients with HCM and a history of paroxysmal AF had lower left atrial emptying fractions than individuals in the control group; and the active atrial emptying fraction was a factor independently associated with the presence of this arrhythmia (p=0.018; odds ratio=0.93). Moreover, we found a significant reduction of the left atrial strain in all its components in the total sample of patients, with no difference between the groups.
ConclusionsMeasurements of atrial emptying fractions by three-dimensional echocardiography allowed differentiating patients with HCM with and without paroxysmal AF.
A miocardiopatia hipertrófica (MCH) é acompanhada de alterações fisiopatológicas que predispõem ao desenvolvimento de fibrilhação auricular (FA). Esta arritmia impacta de forma negativa na morbimortalidade e na qualidade de vida desses doentes. Nosso objetivo foi avaliar o comportamento da função auricular esquerda, por meio do strain auricular (derivado do speckle tracking) e da análise volumétrica pelo ecocardiograma tridimensional, em doentes com MCH portadores de FA paroxística.
MétodoAnalisámos a função auricular esquerda em 53 doentes com MCH, sendo 25 portadores FA paroxística (idade média de 61,7±9,9 anos; 56% sexo feminino) comparados com 28 integrantes do grupo controle (idade média de 60,5±10 anos; 53,6% do sexo feminino) que foram pareados especialmente por sexo, idade e outros dados demográficos.
ResultadosObservou-se que doentes portadores de MCH e antecedentes de FA paroxística apresentaram frações de esvaziamento auricular menores do que indivíduos do grupo controle; sendo que a fração de esvaziamento auricular ativa foi um fator independentemente associado à presença desta arritmia (p=0,018; odds ratio=0,93). Além disso, encontramos redução importante do strain da aurícula esquerda em todos seus componentes na amostra total de pacientes, sem diferença entre os grupos.
ConclusãoAs medidas das frações de esvaziamento auricular pela ecocardiografia tridimensional permitiram diferenciar os doentes com MCH com e sem FA paroxística.
Hypertrophic cardiomyopathy (HCM) is recognized as the most common genetic cardiovascular disease and is associated with a wide range of complications, such as heart failure, atrial fibrillation (AF) with cardioembolic phenomena and, particularly, sudden cardiac death, especially in young individuals.1–3
Atrial fibrillation is considered the most prevalent cardiac arrhythmia in the context of HCM, complicating its natural history in approximately 20% of patients.4,5 Risk stratification for the development of this arrhythmia is of great importance in public health, since its recognition is often delayed and culminates in its main complication, stroke, that is potentially avoided by anticoagulation.6
Patients with HCM are particularly prone to adverse atrial remodeling for many reasons. Among some well-known predisposing factors for atrial remodeling, we can mention the increased left ventricular filling pressures due to diastolic dysfunction, left ventricular hypertrophy, mitral insufficiency, outflow tract obstruction, and atrial fibrosis, that leads to atrial myopathy.7,8
Not all patients with HCM and left atrial (LA) remodeling have AF, and patients can develop it even in the absence of atrial dilation. There is, therefore, a need to characterize, non-invasively, the arrhythmogenic substrate through other indexes, in addition to atrial dilation, in order to improve the prediction of AF in HCM.9
The objective of this study is to compare LA volume and function, speckle tracking technique, in HCM patients with paroxysmal AF (but in sinus rhythm for more than 6 months) and HCM patients who have never had a documented AF episode.
MethodsThis was an observational, case-control, unicentric study, performed through the acquisition of echocardiographic data from patients with HCM with and without paroxysmal AF.
We selected 70 patients seen at the cardiomyopathy clinic from Instituto Dante Pazzanese de Cardiologia, with a confirmed diagnosis of HCM by echocardiogram or cardiac magnetic resonance imaging (CMR). We considered the diagnosis of HCM as an increase in thickness 15 mm in one or more segments of the left ventricular (LV) walls, not explained by other diseases such as aortic stenosis, hypertension or amyloidosis, for example.10
From this total, 35 were in sinus rhythm for at least six months but had previous records of paroxysmal AF on 24-hour Holter, telemetry of the implantable cardioverter-defibrillator (ICD), electrocardiograms performed during emergency care, in an ergometer test or in other outpatient examinations. Patients with HCM without records of AF were matched by sex, age, presence of arterial hypertension (AH), body mass index (BMI), ventricular involvement pattern, and comprised the control group for comparison.
We considered paroxysmal AF any AF episode with spontaneous onset and disappearance within <7 days of onset.11
All individuals recruited to participate in the study protocol were properly guided and authorized their voluntary participation by signing the Term of Consent approved by the Institutional Review Board.
Exclusion criteria for patients in this study were: individuals <18 years old; limited echocardiographic window for image acquisition; patients who had permanent AF or who were at a pacemaker stimulation during exam; uncontrolled arterial hypertension at the time of image acquisition (blood pressure levels >140/90 mmHg); significant mitral regurgitation and mitral stenosis of any degree; left ventricular dysfunction defined by the following criteria: left ventricular ejection fraction (LVEF) <52% for men and 54% for women.12
The echocardiographic images were acquired from December 2018 to January 2020 in a Vivid E9® equipment (GE Vingmed, System, Horton, Norway), equipped with a M5S transducer (sectorial, matrix, broadband frequency) and with 4 V volumetric 3D transducer, according to the recommendations of the American Society of Echocardiography and European Association of Cardiovascular Imaging.12,13 All exams corresponded to complete and comprehensive echocardiographic acquisition, using two-dimensional (2D) and 3D images, pulsed and continuous Doppler, and color flow mapping.
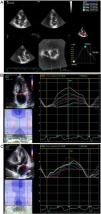
Especially for the assessment of atrial structure and function, 2D images were acquired in the apical views of four and two chambers, with a frame rate between 40 and 80 frames per second, with special attention to the atrial borders, avoiding translational movements of the heart. In addition, a full-volume 3D acquisition, composed of six sub-volumes and with at least 20 frames per second, was also performed. The images were analyzed offline, using the EchoPAC software(GE Healthcare). Supplementary online data describe, in detail, the steps used to measure atrial function using the speckle tracking technique (STE) and using 3D echocardiogram (Figure 1A, 1B and 1C).
A. Example of a three-dimensional technique for evaluation of left atrial function using volumetric reconstruction and obtaining an atrial volume curve in a patient with hypertrophic cardiomyopathy.
LA: left atrium; LV: left ventricle; Vmax: maximum atrial volume; Vmin: minimum atrial volume; VpreA: atrial volume before atrial contraction that precedes the P wave of the electrocardiogram.
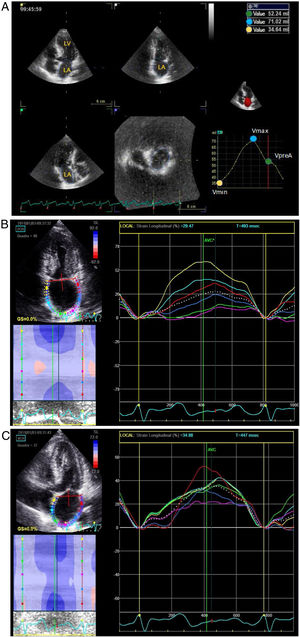
Figure 1B. Example of evaluation of the left atrial function by means of strain analysis performed in a two chamber apical window in a patient with hypertrophic cardiomyopathy.
Figure 1C. Example of evaluation of the left atrial function by means of strain analysis performed in a four chamber apical window in a patient with hypertrophic cardiomyopathy.
Categorical variables of patients were described, according to the presence of paroxysmal AF, using absolute and relative frequencies. Differences between groups were verified using the chi-square tests or exact tests (Fisher's exact test or likelihood-ratio test).
Continuous variables were described, according to the presence of paroxysmal AF, using means and standard deviation, and were compared using student t-tests.14
Pearson correlation test was used to assess the presence of correlations between atrial volumes, emptying fractions and atrial strain with other echocardiographic variables.
Models were created using multiple logistic regression15 to explain the presence of paroxysmal AF according to patients’ characteristics and echocardiograohy parameters that reached statistical significance in the unadjusted analyzes or that have biological plausibility with AF presence.
To perform the analyzes, IBM-SPSS for Windows version 22.0 was used, and Microsoft Excel 2010 (to tabulate the data) were used. Tests were performed with a significance level of 5% (p<0.05).
ResultsSeventy patients were initially selected based on the database of the cardiomyopathy clinic at our institution, of whom 35 patients with paroxysmal AF and other 35 without AF, paired to comprise the control group. Among the 35 patients included in the paroxysmal AF group, 10 patients were excluded from the study for the following reasons: four had an AF rhythm during examination; three had significant mitral regurgitation and three had left ventricular dysfunction.
From the patients eligible for the control group, seven were excluded from the protocol: two due to the limited acoustic window for 3D acquisition; three patients for not accepting the terms established by Consent form and two patients for not attending the visit for echocardiography acquisition. Thus, the final group of patients for analysis was made up of 53 individuals, 25 from the paroxysmal AF group and 28 from the control group.
Table 1 shows the baseline clinical characteristics of patients, comparing them with the control group.
Description of patients’ clinical characteristics according to paroxysmal AF and results of unadjusted statistical tests.
| Variable | Paroxysmal AF | Total (N=53) | p | |
|---|---|---|---|---|
| No (N=28) | Yes (N=25) | |||
| Age (years) | 0.666 | |||
| Mean±SD | 60.5±10 | 61.7±9.9 | 61.1±9.8 | |
| Sex, n (%) | 0.859* | |||
| Female | 15 (53.6) | 14 (56) | 29 (54.7) | |
| Male | 13 (46.4) | 11 (44) | 24 (45.3) | |
| ICD, n (%) | 0.002* | |||
| No | 26 (92.9) | 14 (56) | 40 (75.5) | |
| Yes | 2 (7.1) | 11 (44) | 13 (24.5) | |
| AH, n (%) | 0.572* | |||
| No | 7 (25) | 8 (32) | 15 (28.3) | |
| Yes | 21 (75) | 17 (68) | 38 (71.7) | |
| DLP, n (%) | 0.240* | |||
| No | 6 (21.4) | 9 (36) | 15 (28.3) | |
| Yes | 22 (78.6) | 16 (64) | 38 (71.7) | |
| DM, n (%) | 0.933* | |||
| No | 21 (75) | 19 (76) | 40 (75.5) | |
| Yes | 7 (25) | 6 (24) | 13 (24.5) | |
| SMK, n (%) | 0.430# | |||
| No | 19 (67.9) | 17 (68) | 36 (67.9) | |
| Current | 1 (3.6) | 3 (12) | 4 (7.5) | |
| Former smoker | 8 (28.6) | 5 (20) | 13 (24.5) | |
| Stroke/TIA, n (%) | & | |||
| No | 28 (100) | 25 (100) | 53 (100) | |
| FH of SD, n (%) | >0.999** | |||
| No | 23 (82.1) | 20 (80) | 43 (81.1) | |
| Yes | 5 (17.9) | 5 (20) | 10 (18.9) | |
| SYNCOPE, n (%) | 0.004* | |||
| No | 26 (92.9) | 15 (60) | 41 (77.4) | |
| Yes | 2 (7.1) | 10 (40) | 12 (22.6) | |
| CAD, n (%) | 0.613** | |||
| No | 25 (89.3) | 24 (96) | 49 (92.5) | |
| Yes | 3 (10.7) | 1 (4) | 4 (7.5) | |
| ACEI/ARB, n (%) | 0.637* | |||
| No | 15 (53.6) | 15 (60) | 30 (56.6) | |
| Yes | 13 (46.4) | 10 (40) | 23 (43.4) | |
| BB, n (%) | 0.694** | |||
| No | 3 (10.7) | 4 (16) | 7 (13.2) | |
| Yes | 25 (89.3) | 21 (84) | 46 (86.8) | |
| AMIODARONE, n (%) | <0.001* | |||
| No | 27 (96.4) | 6 (24) | 33 (62.3) | |
| Yes | 1 (3.6) | 19 (76) | 20 (37.7) | |
| STATINE, n (%) | 0.767* | |||
| No | 9 (32.1) | 9 (36) | 18 (34) | |
| Yes | 19 (67.9) | 16 (64) | 35 (66) | |
| ASA, n (%) | 0.060* | |||
| No | 17 (60.7) | 21 (84) | 38 (71.7) | |
| Yes | 11 (39.3) | 4 (16) | 15 (28.3) | |
| WARFARIN, n (%) | <0.001* | |||
| No | 28 (100) | 7 (28) | 35 (66) | |
| Yes | 0 (0) | 18 (72) | 18 (34) | |
| DOAC, n (%) | 0.218** | |||
| No | 28 (100) | 23 (92) | 51 (96.2) | |
| Yes | 0 (0) | 2 (8) | 2 (3.8) | |
| DIURETIC, n (%) | 0.909* | |||
| No | 13 (46.4) | 12 (48) | 25 (47.2) | |
| Yes | 15 (53.6) | 13 (52) | 28 (52.8) | |
| Height (cm) | 0.809 | |||
| Mean±SD | 162.3±9.3 | 161.7±8.7 | 162±8.9 | |
| Weight (kg) | 0.615 | |||
| Mean±SD | 77.7±12.5 | 79.7±16.2 | 78.6±14.3 | |
| BMI (kg/m2) | 0.531 | |||
| Mean±SD | 29.5±4.1 | 30.4±6.4 | 29.9±5.3 | |
| HR in exam | 0.672 | |||
| Mean±SD | 61.2±8.3 | 60.2±8.4 | 60.7±8.3 | |
Student T-test; * Chi-square test; ** Fisher's exact test; # Test of the likelihood ratio; & It is not possible to calculate. SD: standard deviation; ICD: implantable cardioverter defibrillator; AH: arterial hypertension; DLP: dyslipidemia; DM: diabetes mellitus; SMK: smoking; TIA: transient ischemic attack; FH of SD: family history of sudden death; CAD: coronary artery disease; ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; BB: beta-blocker; ASA: acetylsalicylic acid; DOAC: direct oral anticoagulants; BMI: body mass index; HR: heart rate.
In the analysis of the total sample, we found a predominance of women (54.7%) and an average age of 61.1±9.8 years. The average BMI was 29.9±5.3 kg/m2, the average heart rate on the examination was 60.7±8.3 beats per minute and all patients were in sinus rhythm at the time of echocardiographic exam. Patients with paroxysmal AF had a higher prevalence of ICD, a higher incidence of syncope, used more amiodarone and anticoagulation with warfarin (p<0.05).
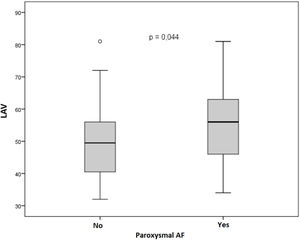
It was possible to observe that patients with paroxysmal AF presented, on average, higher values of LA volume indexed (LAVi) by the body surface area, 2D LA volume (2D LAV) in two chambers, as well as 2D LAV in four chambers view (Figure 2).
In addition, patients in the paroxysmal AF group tended to present lower values of a′ wave measured in the lateral annulus (p=0.050). Tricuspid regurgitation severity was higher in patients with paroxysmal AF (p=0.011).
Regarding the AF measured with a 3D echocardiogram (Table 2), smaller values of active (AAEF) and total atrial emptying fractions (TAEF) were observed in patients with a history of paroxysmal AF (Figure 3). In contrast, such evidence was not seen regarding the passive atrial emptying fraction (PAEF), which had a similar behavior between groups. More information about echocardiographic characteristics can be found in supplementary data.
Echocardiographic variables obtained by the three-dimensional mode and related to the function of the left atrium.
| Variable | Paroxysmal AF | Total (N=53) | p | |
|---|---|---|---|---|
| No (N=28) | Yes (N=25) | |||
| LA Reservoir Strain (%) | 0.588 | |||
| Mean±SD | 18.4±6.4 | 17.3±7.5 | 17.9±6.9 | |
| LA Contraction Strain (%) | 0.259 | |||
| Mean±SD | 10.6±4.8 | 9±5.7 | 9.8±5.3 | |
| LA Coinduit Strain (%) | 0.547 | |||
| Mean±SD | 7.8±3.4 | 8.4±3.4 | 8±3.4 | |
| LAVmax 3D¢ (ml) | 0.481 | |||
| Mean±SD | 79.2±19.3 | 83.4±23.1 | 81.1±21 | |
| LAVmin 3D¢ (ml) | 0.080 | |||
| Mean±SD | 46.7±17 | 56.2±21.4 | 51.1±19.5 | |
| LAVpreA 3D¢ (ml) | 0.181 | |||
| Mean±SD | 60.8±15.2 | 67.6±21.1 | 63.9±18.3 | |
| PAEF¢ (%) | 0.159 | |||
| Mean±SD | 0.228±0.082 | 0.192±0.099 | 0.211±0.091 | |
| AAEF¢ (%) | 0.037 | |||
| Mean±SD | 0.244±0.104 | 0.182±0.105 | 0.215±0.109 | |
| TAEF¢ (%) | 0.013 | |||
| Mean±SD | 0.416±0.093 | 0.339±0.123 | 0.38±0.113 | |
Student T-test; ¢ Not all patients have the measurement. LAVmax 3D: three-dimensional LA maximum volume;
LAVmin 3D: three-dimensional LA minimum volume; LAVpreA 3D: three-dimensional LA pre-atrial contraction volume; PAEF: passive atrial emptying fraction; AAEF: active atrial emptying fraction; TAEF: total atrial emptying fraction.
On the other hand, considering the analysis of strain in all three phases of the atrial cycle (reservoir, conduit and contraction), no difference was observed between groups.
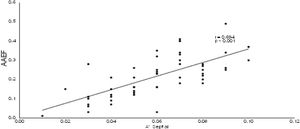
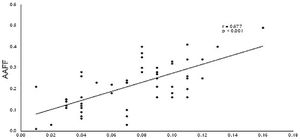
Pearson's correlation tests were carried out and it was observed that active atrial emptying fractions (AAEF) was inversely correlated with the LAVi (r=-0.568; p<0.001), and with the values of atrial volumes acquired by three-dimensional echocardiography (for LAVmax 3D: r=-0.589 with p<0.001; for LAVmin 3D: r=-0.781 with p<0.001; for LAVpreA 3D: r=-0.554 and p<0.001). In addition, there was a direct correlation between AAEF and the speed of a’ wave (for a’ septal’: r=0.694 with p<0.001; for the a’ lateral: r=0.677 and p<0.001), as demonstrated in Figures 4 and 5.
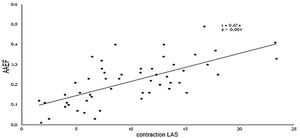
A direct correlation was observed between both methods used in the assessment of atrial function, demonstrating concordance between the values obtained through the AAEF and by the analysis of the atrial contraction strain (Figure 6), as well as in the values of TAEF with atrial reservoir strain (r=0.584 and p<0.001). However,.a significant correlation between PAEF and the conduit strain (r=0.220 and p=0.117) was not demonstrated.
A multivariate analysis of logistic regression was performed, and the presence or absence of paroxysmal AF was considered to be the dependent variable. According to the univariate analysis carried out previously, AAEF was selected as an independent variable, and along with other variables was tested on different models.
Two models were created: Model 1, echocardiographic, included in addition to AAEF variables related to atrial strain. In this model, no variable showed an independent association with paroxysmal AF (supplementary data).
For model 2, clinical, we chose personal characteristics and comorbidities already known in the literature to be associated with the risk of developing AF: age, sex, presence of arterial hypertension and BMI. In this case, in the final model, shown in Table 3, only AAEF remained as a variable independently associated with the presence of paroxysmal AF (p=0.018; odds ratio=0.93). Arterial hypertension was kept in the model since it was close to obtaining statistical significance (p=0.071; odds ratio = 0.22).
Multivariate analysis with final model 2, which tested factors associated with the presence of paroxysmal AF, with odds ratio and their respective confidence intervals (CI 95%) and levels of statistical significance (p).
| Variable | OR | CI (95%) | p | |
|---|---|---|---|---|
| Inferior | Superior | |||
| Age (years) | 1.06 | 0.99 | 1.15 | 0.113 |
| Sex (male) | 0.59 | 0.16 | 2.25 | 0.442 |
| SAH | 0.22 | 0.04 | 1.14 | 0.071 |
| BMI (kg/m2) | 1.04 | 0.92 | 1.18 | 0.535 |
| AAEF (%) | 0.93 | 0.87 | 0.99 | 0.018 |
Multiple logistic regression.
SAH: systemic arterial hypertension; BMI: body mass index; AAEF: active atrial emptying fraction.
The main findings of this study can be summarized as follows: 1) Patients with HCM and paroxysmal AF history presented atrial emptying fractions, measured by 3D echocardiography, smaller than individuals in the control group. 2) AAEF showed an independent association with the presence of paroxysmal AF, when controlled for relevant clinical data. 3) Bidimensional atrial volumes were higher in the paroxysmal AF group, with no difference in the anteroposterior diameter of the LA. 4) There was a significant reduction in LA strain in all its components in the total sample of patients, with no difference between groups.
Left atrium physiology comprises three main components: reservoir function, conduit function and contraction function. The latter seems to be more relevant compared to the others, since this phase basically depends on the intrinsic contractile properties of the LA.8,16 It is evaluated using the LA contraction strain and the AAEF.
The decrease in atrial function and its subsequent structural and electrical remodeling present in HCM can be explained by the changes inherent to the pathophysiology of the disease itself, with decreased left ventricular relaxation and compliance (diastolic dysfunction) that can impair the conduit function, in addition to possible presence of a gradient in the left ventricular outflow tract and mitral regurgitation. Furthermore, myocyte hypertrophy and fibrosis, which are also evident in the atrium of some patients, can lead to impaired atrial relaxation and compliance (reservoir function), elastic atrial recoil (conduit function) and atrial contraction capacity (pump function).4,17
Badano et al.18 obtained LA maximum, minimum and pre-A volumes by means of 3D and 2D echocardiography in 276 healthy volunteers (18-79 years; 57% women). It was evidenced that the normal limits for both volumes and TAEF were higher with the 3D study. From these data, lower limits of normality were established for the atrial emptying fractions, of which: TAEF 53%, PAEF 24% and AAEF 21%.
According to our results, the emptying fractions were reduced in both groups, when compared to this reference, with a greater reduction in the total and active components in the group with paroxysmal AF. Only AAEF remained as a variable independently associated with the presence of paroxysmal AF by multivariate analysis. In addition, there was a direct correlation between AAEF, the atrial contraction strain and the speed of the a’ wave, both denoting negative implications in the active contractile capacity of the LA. It is worth mentioning that literature lacks data related to volumetric analysis and its derived atrial emptying fractions by the three-dimensional method in HCM.
Other authors have already demonstrated that LA volumetric remodeling and the analysis of its phasic function are predictors of AF. Particularly, in agreement with our finding, Maron et al.19 demonstrated that LA maximum volume greater than 118mL and a TAEF less than 38%, measured using CMR in 337 patients, were independent predictors of AF. However, the authors did not assess the other two components of atrial function and their relation as an AF predictor.
Also, Tuluce et al. analyzed the atrial emptying fractions using two-dimensional echocardiogram and showed that TAEF, with a cut-off point of 49%, had a negative predictive value (NPV) of 89% to exclude the presence of AF. In addition, a cutoff point of 36% was found for AAEF, with NPV of 88%.20
Considering the entire sample of patients included in the protocol, we obtained an average of reservoir, contraction and conduit LA strain of 17.9, 9.8 and 8.3%, respectively. These results are well below the normality reference values.21 However, we did not observe any difference between groups.
Perhaps atrial strain is more sensitive to assess atrial function than three-dimensional echocardiography. In fact, in our study both groups showed a marked reduction in function measured by strain, compared to normal values. Therefore, it is possible that due to the small number of patients analyzed, the difference between groups was too small to be detected. On the other hand, 3D-phased measurement of volumes may be a more specific technique and, therefore, may have been able to show the difference between groups, with an additional independent association of AAEF and paroxysmal AF.
Debbonaire et al.22 in a study on the hypertrophic population, concluded that patients with LA reservoir strain (LASR) <23.4% and LA conduit strain <10.2% had lower AF free survival than the control group paired by sex and age, but not adjusted for comorbidities.
It is already recommended by the current guidelines to intensify surveillance for AF in HCM patients, indicating 48-hour Holter every 6 months after evidence of LA diameter ≥45 mm. However, we know that atrial enlargement in the anteroposterior direction is restricted by the presence of the sternum and mediastinum and, consequently, its remodeling is not uniform in all directions.9 In our study, we found no difference between the groups when analyzing only the anteroposterior LA diameter, which reinforces the importance of volumetric measurements of the atrium, particularly those obtained by the three-dimensional echocardiography.
After a comprehensive literature review, our study seems to be the first to demonstrate AF changes in LA volume and function in patients with HCM, using 3D volumetric analysis and LA strain.
One of the limitations of this study is the small number of patients comprising the sample. This is due especially to the low prevalence of HCM in the general population, and the fact that our institution is a tertiary care hospital, being difficult to recruit patients without previous treatment approaches (such as myectomy, septal ablation, among others) and who were not diagnosed with persistent/permanent AF.
In summary, our data suggest that reduction in LA function, obtained by the analysis of volumetric variations and emptying fractions by means of three-dimensional echocardiography, could help identifying patients with HCM at risk of developing AF. Therefore, these echocardiographic parameters should be integrated into the assessment of patients with HCM, in order to reduce the burden of this arrhythmia on this population.
However, we recognize that the case-control nature of our study does not allow defining the predictive capacity of AAEF in predicting AF. We believe that additional studies, especially prospective cohorts, are needed to confirm this finding.
ConclusionPatients with HCM and paroxysmal AF show structural remodeling of the left atrium associated with an important impairment of its function. Comparing them to patients with HCM and without a history of paroxysmal AF, we observed that there is a greater impairment of atrial function by the volumetric variation and emptying fractions, obtained by the 3D method. In addition, AAEF was independently associated with the presence of paroxysmal AF, when controlled by age, sex, hypertension, and BMI.
Conflicts of interestThe authors have no conflicts of interest to declare.