Pulmonary embolism due to primary antiphospholipid syndrome is rarely associated with chronic thromboembolic pulmonary hypertension, and therefore according to the latest guidelines on pulmonary hypertension, routine screening is not recommended. We describe a young patient with a late diagnosis of chronic thromboembolic pulmonary hypertension in the context of pulmonary embolism, primary antiphospholipid syndrome and suboptimal anticoagulation. Of note, mild cardiopulmonary symptoms were consistently misattributed to a depressive disorder because physical examination was normal, serial Doppler echocardiography failed to show pulmonary hypertension, and all other diagnostic tests were normal. Once symptoms became severe, positive screening tests led to the correct diagnosis and surgical referral, and bilateral pulmonary endarterectomy was successfully performed. This case demonstrates the need for extra awareness in patients with antiphospholipid syndrome and pulmonary embolism.
A embolia pulmonar devido à síndrome de anticorpo antifosfolípido raramente está associada a hipertensão pulmonar crónica tromboembólica, pelo que o seu rastreio não está recomendado pelas normas de orientação clínica atuais. Descreve-se o caso de uma doente jovem com o diagnóstico tardio de hipertensão pulmonar crónica tromboembólica no contexto de síndrome de anticorpo antifosfolípido primário e anticoagulação subterapêutica. A destacar que a sintomatologia cardiopulmonar de grau ligeiro foi incorretamente atribuída a humor depressivo devido à ausência de alterações no exame objetivo e nos meios complementares de diagnóstico, incluindo valores persistentemente normais de pressão sistólica da artéria pulmonar nos ecocardiogramas transtorácicos seriados. O agravamento sintomático progressivo conduziu à confirmação diagnóstica, após realização dos meios complementares de diagnóstico de rastreio, referenciação cirúrgica e realização de endarterectomia pulmonar bilateral com sucesso. Este caso demonstra a necessidade de uma vigilância mais apertada em doentes com síndrome de anticorpo antifosfolípido e embolia pulmonar.
Pulmonary embolism (PE) due to primary antiphospholipid syndrome (APS) may be associated with chronic thromboembolic pulmonary hypertension (CTEPH), a form of pulmonary hypertension (PH) characterized by impaired dissolution of thrombi due to pulmonary embolism (PE) persisting beyond 3-6 months of adequate anticoagulation.1–4 CTEPH is a life-threatening condition of unpredictable onset,5 and, in the setting of APS, may occur soon after the thrombotic event.6 We report a young patient in whom CTEPH was diagnosed late in the course of the disease, with a favorable therapeutic outcome. Notwithstanding its rarity, we aim to raise awareness of the possibility of PH development in patients with APS.
Case reportIn 1993, a previously healthy 20-year-old nulliparous female suffered a PE secondary to an unprovoked peripheral deep vein thrombosis (DVT), after which she was treated with warfarin for nine months. Three months later she suffered a second DVT. At this time inherited thrombophilia disorders were excluded, lupus anticoagulant was persistently positive and she was diagnosed with primary APS. Despite a recommendation for life-long anticoagulation with warfarin, she only adhered to the therapy for six months.
In 2003 (aged 30), she was referred to our unit. Although she was not taking any medication, no further thromboembolic episodes had occurred. Nevertheless, she was suffering from anorexia, anxiety and depression, and had been prescribed fluoxetine 20 mg/day. Warfarin was restarted. In 2006 (aged 33), 13 years after the PE, she began complaining of asthenia, fatigue and mild effort dyspnea, but there were no relevant findings on physical examination. Full blood count and liver and renal function tests were normal; transthoracic echocardiography (TTE) showed no anatomical or functional changes, including normal estimated pulmonary artery systolic pressure (PASP) on Doppler TTE; thoracic computed tomography (CT) revealed no signs of interstitial lung disease. Over the following years her symptoms slowly increased in severity and she was unable to work. Retrieved monthly international normalized ratio records ranged between 2.0 and 4.0 after 2006. The pattern in serial TTEs remained unchanged and her symptoms were consistently attributed to persistent depression.
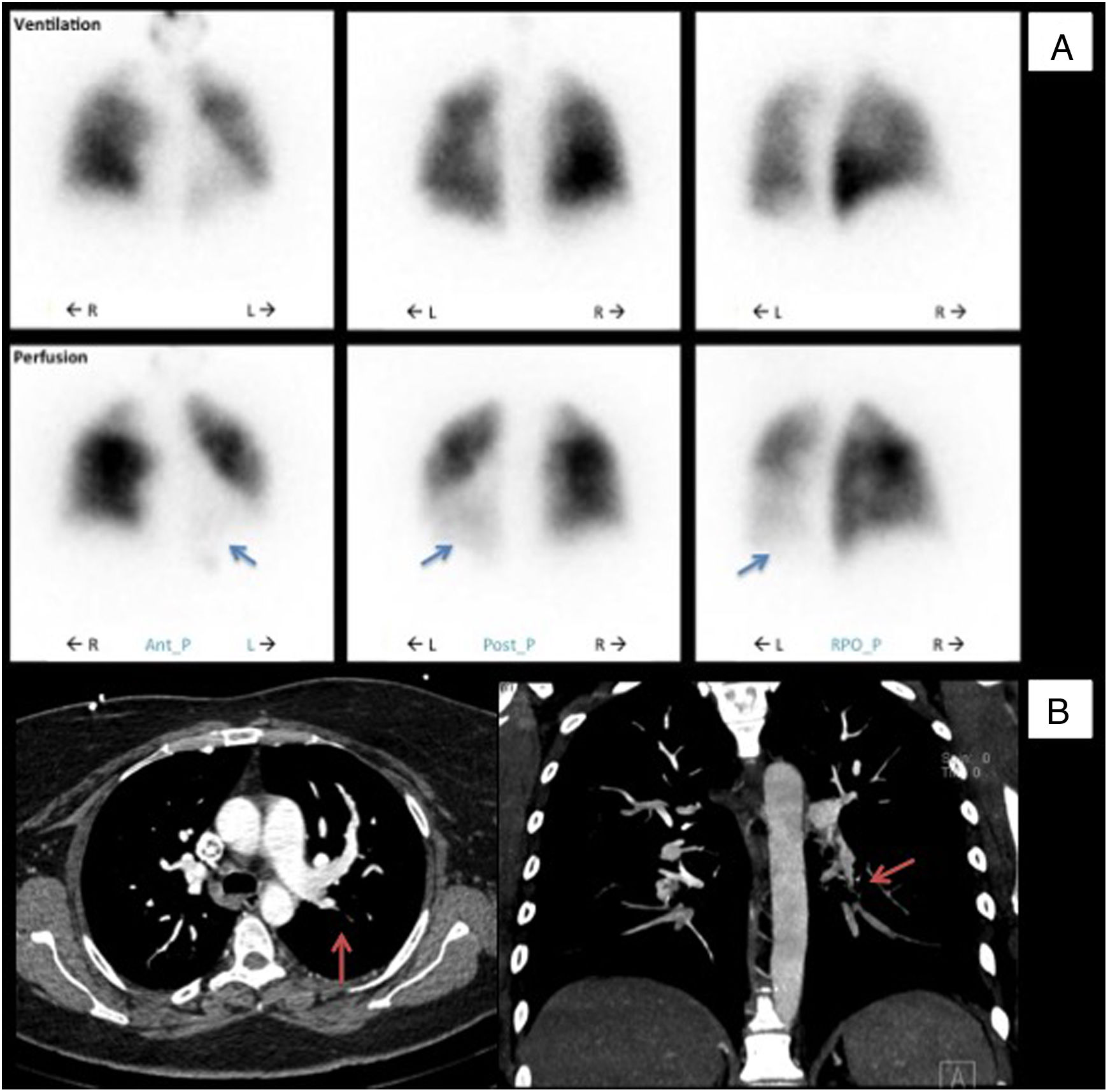
In 2013 (aged 40), at a routine follow-up appointment, she complained of severe exertional dyspnea and palpitations. Physical examination revealed central cyanosis and tachycardia, and peripheral capillary oxygen saturation (SpO2) was under 90%. Blood tests showed polycythemia and elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP). Doppler TTE showed an estimated PASP of 100 mmHg (normal <35 mmHg7) and she was promptly referred to a specialist pulmonary hypertension center. Further investigations were as follows: pulmonary function tests were normal; the six-minute walk test (Table 1) revealed normal chronotropic and inotropic competence on exertion but reduced functional performance (75% of normal)8; the pulmonary ventilation/perfusion scan (Figure 1A) indicated subsegmental perfusion defects with preserved ventilation of all the left lobe segments and of the superior and posterior basal segments of the right lobe; CT angiography (Figure 1B) showed a subocclusive stenotic lesion at the origin of the left inferior lobar artery and bilateral involvement of lobar and peripheral branches; right heart catheterization (Table 1) disclosed low cardiac output and index, elevated mean pulmonary artery pressure, pulmonary vascular resistance three times the upper limit of normal, and pulmonary artery wedge pressure below the threshold, overall indicative of moderate precapillary PH; and pulmonary angiography (Video 1) showed multiple filling defects with total occlusion of the left inferior lobar artery in addition to several segments of the right superior and inferior lobes.
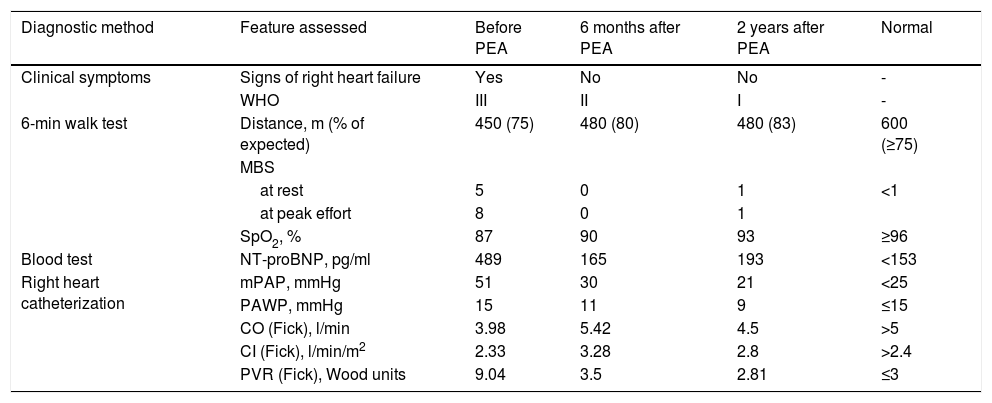
Characterization of parameters and hemodynamics before and after pulmonary endarterectomy.
| Diagnostic method | Feature assessed | Before PEA | 6 months after PEA | 2 years after PEA | Normal |
|---|---|---|---|---|---|
| Clinical symptoms | Signs of right heart failure | Yes | No | No | - |
| WHO | III | II | I | - | |
| 6-min walk test | Distance, m (% of expected) | 450 (75) | 480 (80) | 480 (83) | 600 (≥75) |
| MBS | |||||
| at rest | 5 | 0 | 1 | <1 | |
| at peak effort | 8 | 0 | 1 | ||
| SpO2, % | 87 | 90 | 93 | ≥96 | |
| Blood test | NT-proBNP, pg/ml | 489 | 165 | 193 | <153 |
| Right heart catheterization | mPAP, mmHg | 51 | 30 | 21 | <25 |
| PAWP, mmHg | 15 | 11 | 9 | ≤15 | |
| CO (Fick), l/min | 3.98 | 5.42 | 4.5 | >5 | |
| CI (Fick), l/min/m2 | 2.33 | 3.28 | 2.8 | >2.4 | |
| PVR (Fick), Wood units | 9.04 | 3.5 | 2.81 | ≤3 | |
CI (Fick): cardiac index by Fick's method; CO (Fick): cardiac output by Fick's method; MBS: modified Borg scale; mPAP: mean pulmonary arterial pressure; NT-proBNP: N-terminal pro-B-type natriuretic peptide; PASP: pulmonary artery systolic pressure; PAWP: pulmonary artery wedge pressure; PEA: pulmonary endarterectomy; PVR (Fick): pulmonary vascular resistance by Fick's method; SpO2: peripheral capillary oxygen saturation; WHO: World Health Organization functional class.
Initial diagnostic procedures. (A) Pulmonary ventilation/perfusion scan with subsegmental perfusion defects (blue arrows) and preserved ventilation in the left lower lobe; (B) computed tomography angiography with a subocclusive stenotic lesion at the origin of the left inferior lobar artery (red arrows). Ant_P: anterior-posterior; L: left; Post-P: postero-posterior; R: right; RPO_P: right posterior oblique-posterior.
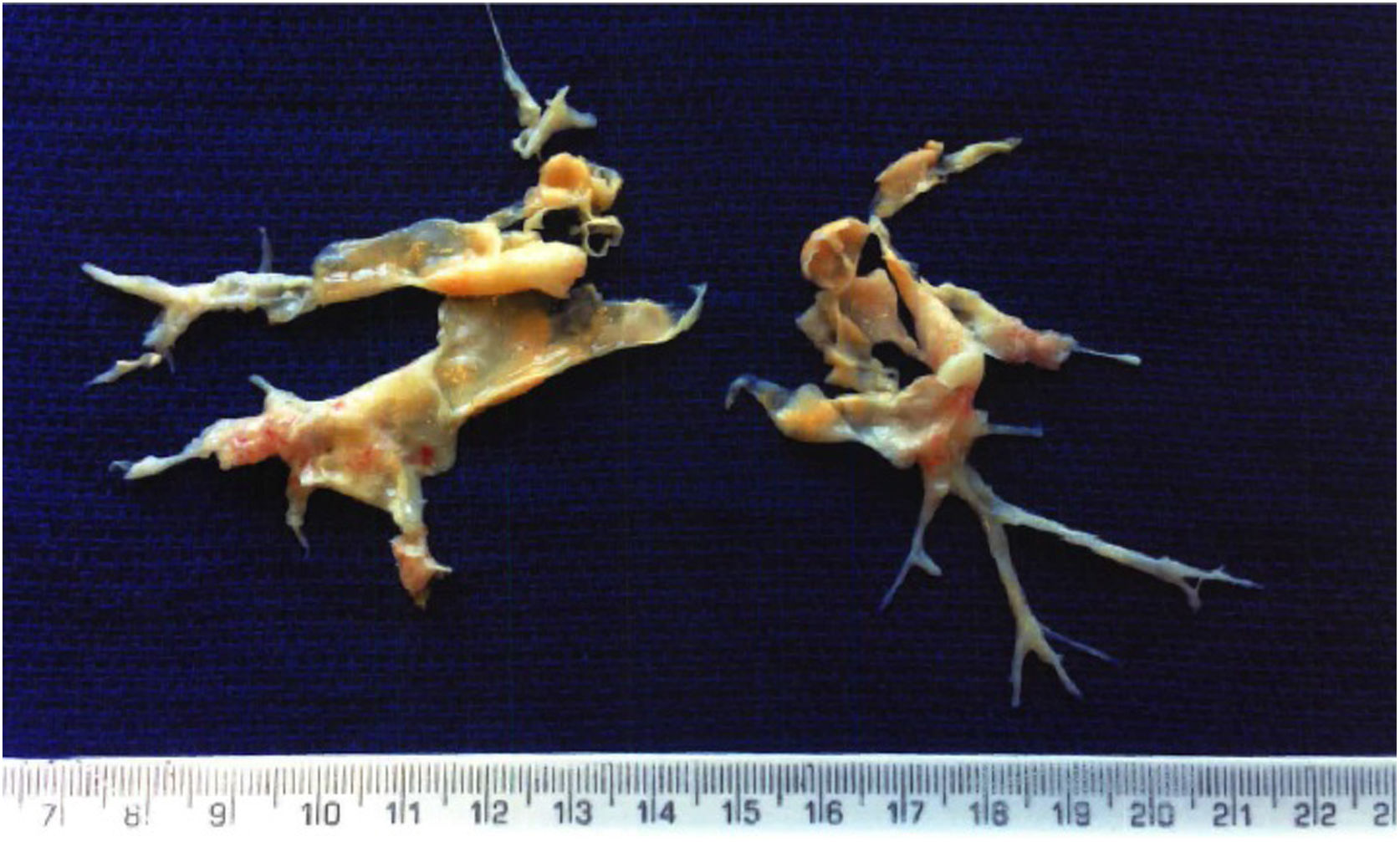
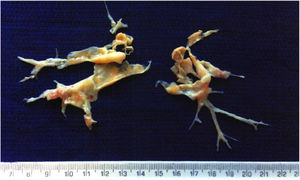
Pulmonary endarterectomy was performed through a median sternotomy approach at an international reference center for CTEPH in March 2015, with full bilateral removal of thrombi and fibrotic material (Figure 2). There were no postoperative complications and follow-up tests (Table 1) showed improvements in all parameters, most noticeably WHO functional class, six-minute walk distance, biochemical markers (NT-proBNP) and hemodynamic parameters on right heart catheterization.
At the present time, more than two years after surgery, the patient is asymptomatic (WHO functional class I). Furthermore, her quality of life has improved significantly, allowing her to keep a full-time job. She remains under lifelong oral anticoagulation with warfarin without need for additional pulmonary vasodilator therapy.
Discussion and ConclusionsCTEPH is a rare disorder with a reported prevalence of 3 and 63 per million individuals in Europe and in the USA, respectively.9,10 Its incidence is low (reported as 0.57%11 and 4-5%12 after PE), and as it can occur up to two years after an acute thrombotic episode,1,6 routine screening for CTEPH after PE is not supported by current evidence.4 Thrombus size, immune-mediated mechanisms, elevated factor VIII, thyroid replacement, structural cardiac abnormalities and malignancy have also been reported as being associated with CTEPH.3,13–15 However, its etiology remains unclear, as it may develop in the absence of PE,16,17 despite adequate anticoagulation and in association with generalized pulmonary vasculopathy.1
Of note, our patient was asymptomatic for 12 years after the last thrombotic event. Although cardiopulmonary symptoms were observed, their insidious nature led to misattribution to a depressive disorder. Detection of PH by TTE remains a challenge18,19 and we recognize that the patient might have benefited from an earlier diagnostic work-up. Moreover, she had various known risk factors for CTEPH, including several thrombotic episodes, a background of autoimmunity and a prolonged period of inadequate therapy, which collectively may have contributed to impaired thrombus resolution. Pulmonary endarterectomy is a complex surgical procedure, but it not only arrests the malignant natural course of the disease that results in premature mortality but also leads to hemodynamic and functional improvement.1,2,5,20 Our patient requires life-long monitoring and anticoagulation, as there are no known prognostic markers that indicate the possibility of disease recurrence.
In conclusion, CTEPH is rare but fatal if left untreated and there should therefore be a high level of suspicion for non-specific symptoms, despite the presence of depression and especially when there is a history of previous venous thromboembolism.20 APS and failure to adhere to anticoagulant therapy should raise the bar for CTEPH screening.
Conflicts of interestThe authors have no conflicts of interest or financial disclosures to declare.
Mr. David Jenkins, Clinical Director of Surgery and Consultant Cardiothoracic Surgeon at the Papworth Hospital NHS Foundation Trust, Cambridge, United Kingdom, was responsible for the pulmonary endarterectomy.