Coronary artery perforation (CAP) is a rare but feared complication of percutaneous coronary intervention. With the advent of new devices and technologies, interventionalists attempt more complex lesions, including more calcified or tortuous vessels and chronic total occlusions, which increases the incidence of CAP. A short literature review, in addition to four cases of CAP, is presented in this report.
As perfurações das artérias coronárias são raras, mas complicações temidas nas intervenções coronarianas percutâneas. Com o advento de novos dispositivos e tecnologias, a tentativa de intervenção em lesões mais complexas, incluindo os vasos mais calcificados ou tortuosos ou oclusões crónicas, leva a maior incidência de perfurações de artérias coronárias. Uma breve revisão da literatura, além de quatro casos de perfurações de artérias coronárias foi apresentada no presente trabalho.
Coronary artery perforation (CAP) is a rare but potentially life-threatening complication of percutaneous coronary intervention (PCI). Its incidence depends on the material and methods for visualizing or opening the coronary arteries. With the advent of new devices and technologies, interventionalists attempt more complex lesions, including more calcified or tortuous vessels.
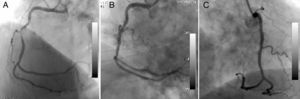
The first patient was a 70-year-old man complaining of chest pain despite medical treatment. Coronary angiography (CAG) was performed electively with a diagnosis of stable angina pectoris. CAG revealed a long, angulated, eccentric critical lesion in the mid right coronary artery (Figure 1A). Direct stenting of the target lesion was performed, and then a type I coronary perforation (limited to the vessel wall without extravasation) was detected on the angiogram (Figure 1B). Prolonged balloon inflation only was applied to the ruptured area and the control angiogram showed no extravasation (Figure 1C). There was no pericardial effusion on transthoracic echocardigraphy after the procedure.
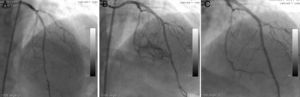
The second patient was a 58-year-old woman who went to the cardiology outpatient clinic with new-onset, progressive, oppressive chest pain, diagnosed as unstable angina. A long, calcified, eccentric critical lesion was detected in the mid to distal left anterior descending (LAD) coronary artery during CAG (Figure 2A). Stenting of the target lesion was performed after balloon predilatation. A type II perforation (showing limited extravasation with some myocardial blushing) was seen on CAG (Figure 2B). A covered stent was immediately implanted to cover the rupture and anticoagulation was reversed. No myocardial blushing was seen on the control CAG (Figure 2C). A mild pericardial effusion, not causing tamponade, was detected by echocardigraphy.
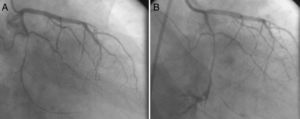
The third patient was a 47-year-old man, who was referred to the cardiology clinic with chest pain on exercise. Since exercise stress testing showed 3-mm horizontal ST-segment depression at the target heart rate, CAG was scheduled. A long, thin, eccentric and calcified critical lesion was seen in the mid circumflex coronary artery during CAG (Figure 3A). Stenting after balloon predilatation was planned, but a type III coronary rupture (demonstrating significant contrast streaming into the pericardium) was observed while the guidewire was being advanced (Figure 3B). Because a covered stent of suitable size for such a thin lesion was not available in the catheterization laboratory, the patient underwent urgent surgical repair with prolonged balloon inflation proximal to the ruptured area to prevent cardiac tamponade. In addition, reversal of anticoagulation therapy was achieved by protamine.
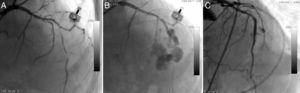
The fourth patient was a 74-year-old woman who went to the emergency department with new-onset severe chest pain and was diagnosed with acute anterior myocardial infarction. She was transferred to the catheterization laboratory for primary PCI. Long, thin, angulated and eccentric critical sequential lesions with thrombus were detected in the mid to distal portion of the LAD (Figure 4A). After stenting followed by balloon predilatations, prominent contrast flow into the left ventricle was detected on CAG, indicating type IV coronary rupture (Figure 4B). A covered stent was immediately implanted in the ruptured area and anticoagulation was reversed. Control CAG showed no extravasation around the target area (Figure 4C). The echocardiogram revealed mild pericardial effusion with no evidence of cardiac tamponade.
Long, thin, angulated and eccentric critical sequential lesions with thrombus in the mid to distal portion of the left anterior descending coronary artery (A); type IV coronary rupture showing prominent contrast flow into the left ventricle (B); control angiogram showing no extravasation around the target area after covered stent implantation (C).
The hemodynamic parameters of all of these patients were within normal limits during follow-up and they were discharged fully recovered from the hospital.
ReviewCAP is a rare but feared complication of PCI. Its incidence varies according to patient, lesion and procedure characteristics; studies have reported incidences ranging from 0.29 to 3.0%.1,2 Risk increases with the complexity of the lesions, including chronic total occlusions, angulated calcified type B2 and type C lesions, long (>10 mm), eccentric lesions, and small vessel size.1–3 Older age and previous coronary artery bypass graft surgery also increase the risk.1,4,5 Risk factors include conditions associated with increased calcification such as diabetes, hypertension and chronic renal failure.4,6 Although females are thought to be more prone to perforation due to their smaller vasculature, the data are inconsistent.7,8
Ellis et al. classified coronary perforations based on their angiographic appearance.1 Type I perforations are limited to the vessel wall and produce an intramural crater without extravasation on the angiogram. In contrast to type I, types II and III are not limited to the vessel wall. Type II perforations show limited extravasation with pericardial or myocardial blushing on angiography, whereas in type III prominent contrast streaming from a ≥1-mm tear is seen. In the cavity spilling subtype (type IV for some authors) contrast flow can be seen from the perforation site into a cardiac chamber or cavity, such as the left ventricle or coronary sinus, rather than into the pericardium or myocardium. Muller et al. proposed adding a type V to the classification, describing distal perforation associated with the use of hydrophilic and/or stiff guides.9 Although other classifications are used, Ellis’ is the most widely accepted.2,10,11
CAP may occur with the use of guiding catheters, guidewires, oversized balloon/stents, cutting balloons, intravascular ultrasound (IVUS) catheters, or debulking techniques, or following balloon rupture.3,12,13 A significant proportion of perforations occur with guidewires crossing the lesion, with distal wire perforation or wire fracture.14 Physical features of the wire affect the likelihood of CAP. In general, stiffer guides increase the risk of perforation. Likewise, hydrophilic-coated wires have been found to be associated with CAP in some series.15,16 However; this may reflect either use of these low-friction hydrophilic coated wires to facilitate passage through more complex lesions or their ease of distal migration.17
Perforations occur more frequently with debulking techniques than with non-debulking techniques. Considering the mechanism of vessel injury, atheroablative devices often cause type III perforations.18 Perforation is more likely when IVUS is used, probably because IVUS is frequently used for complex lesions or when PCI is complicated.19 Whether concomitant administration of GP IIb/IIIa inhibitors increases the likelihood of CAP is controversial, since some studies show increased perforation while others do not.4,19–21 This may be a selection bias, since these agents are generally used in more complicated conditions, but another possible explanation is that they may unmask a minimal vessel tear and convert it to overt perforation.21
Therapeutic strategies include prolonged balloon inflation, covered stents, reversal of anticoagulation, embolization of the distal vessel and surgery, the choice depending on the site and severity of the perforation, the patient's hemodynamic status and the equipment available in the catheterization laboratory.8 Echocardiography should be performed as soon as a perforation is identified. If pericardial hemorrhage or hemodynamic collapse occur pericardiocentesis should be performed immediately with multiple side holes for continuous aspiration, after which the drain should be kept in place for 6–24 hours and reaccumulation should be monitored with echocardiography.12 Administration of fluids is recommended. A balloon should immediately be placed with inflations lasting up to 5-10 minutes to block extravasation. If the perforation cannot be sealed, repeated inflations should be made. Distal ischemia being a concern, perfusion balloons can be used without blocking distal blood flow. Reversal of anticoagulation can be achieved with protamine. As previously shown, the use of protamine is safe and does not predispose to stent thrombosis.14,22 However, diabetic patients on protamine containing insulin and patients with fish allergy are at increased risk for protamine reactions. GP IIb/IIIa inhibitors should also be discontinued and platelet transfusions should be used if needed.
Deployment of a covered stent is another therapeutic approach, especially with a large tear involving a proximal or mid coronary artery. Autologous vein-covered stents have been used successfully in the past,23,24 but this technique is time-consuming and requires expertise. Polytetrafluoroethylene (PTFE) is an inert and biocompatible polymer composed of carbon chains saturated with fluorine and in contrast to vein-covered stents, PTFE-covered stents are easy and rapid to deploy. However, due to the high profile and poor flexibility of these stents, it is often difficult to deliver them to the target site, especially when the vessel is heavily calcified and tortuous.25 The use of IVUS to ensure correct stent implantation and final high-pressure balloon inflation may improve the outcome.26 Pericardium-covered stents with greater flexibility are an alternative treatment, although experience is limited.27 Stent thrombosis and in-stent restenosis are major concerns with covered stents, as is side branch occlusion.19,25 Although data are scarce, prasugrel, due to its lack of intrinsic resistance, can be considered the thienopyridine of choice for stent thrombosis.28 Another major drawback, the time elapsed between deflation of the sealing balloon and the final delivery of the covered stent to the lesion site, can be overcome by a dual catheter technique.29
Alternative therapies used in selected cases include coil embolization, thrombogenic particles including polyvinyl alcohol, gelfoam, thrombin, embolic agents like N-butyl cyanoacrylate glue, and autologous blood clot.30,31 Although potentially useful in emergency situations, these agents carry a risk of loss of the vessel lumen and subsequent infarction. Vessel occlusion techniques should therefore be used as a last resort for the treatment of distal perforations, in which the potential for myocardial injury is limited.30
If a large perforation causes severe ischemia or hemodynamic deterioration or cannot be sealed with the available techniques, emergency surgery is indicated. Surgical intervention may be life-saving, but, since these patients have more severe perforations, it is associated with higher morbidity and mortality and worse outcome. Earlier surgical referral should be considered in this context when dealing with high-grade perforations.6
ConclusionsCAP is a rare but feared complication in the catheterization laboratory. PCI in calcified or tortuous vessels or in chronic total occlusions or complex lesions increases the risk of CAP. Choosing appropriate therapy may be life-saving.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.