Chronic aortic regurgitation (AR) is a valvulopathy of slow and insidious evolution, and patients may remain asymptomatic for a long period of time. Exercise-induced systolic dysfunction occurs during the natural history of chronic AR and is related to changes in both preload and afterload. We describe the case of a 58-year-old woman with a diagnosis of chronic AR who reported progressive dyspnea of six years’ duration. A cardiopulmonary exercise test to assess functional capacity showed flattening of both oxygen uptake and oxygen pulse curves, suggesting latent systolic dysfunction related to chronic AR, which was later confirmed by stress Doppler echocardiogram with dynamic physical exercise.
A Insuficiência Aórtica Crônica (IAC) é uma valvulopatia de evolução lenta e insidiosa podendo o paciente manter-se assintomático por longo período de tempo. A disfunção sistólica induzida pelo esforço ocorre durante a história natural da IAC estando relacionada com alterações nos componentes tanto da pré-carga quanto da pós-carga. Apresentamos o caso de uma paciente do género feminino, de 58 anos, com diagnóstico de IAC que referia queixa de dispneia progressiva com evolução de seis anos. O teste cardiopulmonar foi indicado com intuito de avaliar a capacidade funcional e mostrou platô das curvas de consumo de oxigénio e de pulso de oxigénio, sugerindo disfunção sistólica latente relacionada à insuficiência aórtica crônica; condição posteriormente confirmada pelo Doppler-ecocardiograma de estresse com esforço físico dinâmico.
Cardiopulmonary exercise testing (CPET) is well established as a useful tool for the evaluation of functional capacity in various forms of heart disease.1 Among the commonly assessed variables, peak oxygen uptake (VO2) is extensively used for the serial evaluation of patients with heart failure who are candidates for heart transplantation.2 Emerging variables such as VE/VCO2 slope and periodic breathing are valuable for the prognostic evaluation of patients with symptomatic ventricular dysfunction.3 Oxygen pulse, defined as the ratio of oxygen uptake to heart rate and expressed as ml/bpm, is closely related to stroke volume, and is used as a parameter for the assessment of systolic performance.4 It is thus a potential variable for the diagnosis of heart disease that follows a similar course with systolic dysfunction during exercise. However, this diagnostic application of CPET continues to be underutilized.
We report the case of a 58-year-old woman with a diagnosis of chronic aortic regurgitation (AR) in whom the behavior of CPET variables indicated the presence of latent left ventricular (LV) systolic dysfunction. This diagnosis was confirmed by echocardiographic findings during exercise stress.
Case reportA 58-year-old black woman, BMI 33.2 kg/m2 (weight 71.8 kg; height 1.47 m), with a history of hypertension and under regular treatment with losartan potassium, hydrochlorothiazide and pantoprazole, reported a history of progressive exertional dyspnea of six years’ duration and resting dyspnea and episodes of nocturnal paroxysmal dyspnea during the last few months. Physical examination revealed an active, eupneic, afebrile, acyanotic, anicteric patient with pink and moist mucous membranes and without jugular distension. Pulmonary auscultation revealed no abnormalities in either lung field. Cardiomegaly was absent and cardiac rhythm was regular with normal heart sounds, but an early-mid-diastolic respiratory murmur was present over the aortic area (2+/6+). Blood pressure was 140/60 mmHg and heart rate was 76 bpm.
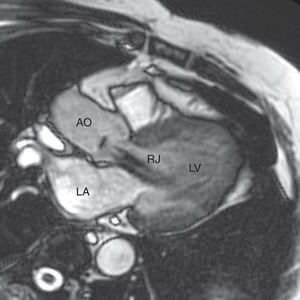
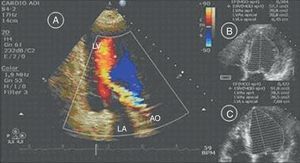
A conventional resting electrocardiogram showed sinus rhythm, heart rate of 78 bpm and left ventricular overload. A chest X-ray revealed a normal cardiothoracic index and no signs of pulmonary congestion. A resting Doppler echocardiogram revealed aortic regurgitation, defined as moderate based on the slope of the deceleration curve in diastole (slope 354 cm/s2) and no holodiastolic retrograde blood flow in the abdominal aorta (score 6). The eccentricity of the regurgitant jet limited the possibility of using the width and area of the aortic regurgitation jet as parameters to classify the severity of the valvulopathy (Figure 1A). There was eccentric LV hypertrophy but no signs suggestive of pulmonary arterial hypertension. The following chamber diameters and volumes were calculated: left atrial diameter 38 mm, end-diastolic LV diameter 64 mm, end-systolic LV diameter 42 mm, end-diastolic LV volume 142 ml, and end-systolic LV volume 57 ml. Global systolic function defined by ejection fraction as calculated by Simpson's method was 59%. Peak and mean aortic valve gradients were not calculated (Figure 1B).
In view of this discrepancy between the symptoms reported by the patient and the echocardiographic findings, CPET was scheduled in order to obtain an objective assessment of the patient's functional capacity. Using the Balke protocol, with a fixed speed of 2.0 mph and 2% slope increments per minute, the maximum work achieved by the patient was 14% inclination at 2.0 mph. The patient's heart rate showed a physiological behavior during exercise, starting at a resting heart rate of 78 bpm and reaching 141 bpm at peak exercise (87% of maximum predicted heart rate). Similarly, the behavior of blood pressure during the test was normal, starting exercise at a resting blood pressure of 130/70 mmHg and reaching 175/70 mmHg at peak exercise. Throughout the test, the electrocardiogram showed no changes in ventricular repolarization suggestive of myocardial ischemia.
The following maximum values of the conventional CPET variables were obtained: minute ventilation (VE) 32.7 l/min, VO2 847.2 ml/min, peak carbon dioxide production (VCO2) 886.2 ml/min, and respiratory exchange ratio 1.07. The ventilatory anaerobic threshold was identified at 6 minutes 16 seconds at a speed of 2.0 mph and 4% inclination, with VO2 of 10.6 ml/kg/min at a heart rate of 105 bpm and blood pressure of 145/70 mmHg.
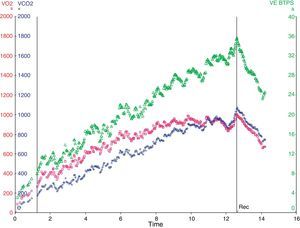
During the initial phase of exercise, VE and VO2 increased linearly; however, the VO2 curve reached a plateau after the sixth minute, whereas VE maintained a linear increase (Figure 2).
Heart rate showed a linear increase proportional to the increase in work, whereas oxygen pulse showed a plateau behavior with a slight decline at peak exercise suggestive of a fall in LV ejection volume during exercise. The highest oxygen pulse value reached during exercise was 8 ml/systole (Figure 3). It should be pointed out that normal values range from 4 to 6 ml/systole at rest, but can reach 10–20 ml/systole at peak exercise.4
In order to confirm the role of systolic dysfunction during exercise as the cause of the plateau followed by a fall in oxygen pulse during CPET, a Doppler echocardiographic study during dynamic physical exercise was performed using the same exercise protocol employed for CPET. Under these conditions, the following results were obtained at peak exercise compared to rest: increases in LV end-diastolic diameter from 64 to 68 mm, in LV end-systolic volume from 57 to 91 ml, and in end-diastolic volume from 142 to 159 ml, and a fall in ejection fraction from 59 to 42% (Figure 1C). LV ejection fraction was also evaluated by radioisotope ventriculography at rest and during dynamic physical exercise, with 61% being observed at rest and 47% at peak exercise.
Cardiac MRI was also performed for comparison with the Doppler echocardiographic study in order to estimate the severity of aortic regurgitation, with the following results: left atrial diameter 40 mm, end-diastolic volume 114.3 ml, end-systolic volume 47.3 ml, LV ejection fraction 58.6%, anterograde flow 46.5 ml, retrograde flow 39.0 ml, and regurgitant fraction 84% (Figure 4). These cardiac MRI parameters characterize the aortic regurgitation as severe.
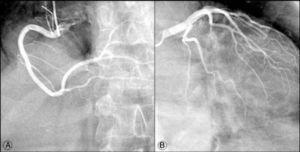
Cardiac catheterization (Figure 5) demonstrated coronary artery circulation free of obstructive lesions and cineventriculography revealed normal LV segmental wall motion.
DiscussionWe report a clear discordance in a patient with chronic AR between the clinical manifestations and the estimated severity of valvular heart disease evaluated by initial resting Doppler echocardiographic study. In this clinical context, it is extremely important to characterize the symptoms meticulously, especially in patients who complain of dyspnea, in order to avoid over-hasty surgical indication for patients with symptoms that may not be due to valve damage.5
Chronic AR influences the dynamics of homeostasis of the circulating intravascular volume and can lead to a redistribution of body fluids that may explain the nocturnal paroxysmal dyspnea that can occur as an isolated symptom in low functional class patients. Dyspnea is a subjective symptom that is multifactorial in nature and the patient's sedentarism, obesity and low cardiorespiratory fitness definitely influenced the sensation of resting dyspnea she reported.6
Symptoms of progressive dyspnea may be attributed to heart failure with normal ejection fraction (“diastolic heart failure”) secondary to hypertension and LV hypertrophy. However, the patient did not fulfill criteria of heart failure, with a normal ejection fraction being observed on Doppler echocardiographic study.7 Type B natriuretic peptide was not measured.
In view of the large number of clinical conditions that can influence oxygen pulse, especially pulmonary disease, we performed spirometry, which revealed no ventilatory disorder with an obstructive or restrictive pattern.
In this case, analysis of CPET ventilatory variables demonstrated significant limitation of functional capacity, reflected by low maximum oxygen uptake (peak VO2 of 11.8 ml/kg/min) associated with flattening of the VO2 curve. In addition, the oxygen pulse curve showed a plateau followed by a fall at peak dynamic physical exercise. Taken together, these findings demonstrate impaired LV systolic function during exercise, indicating a severe functional repercussion of aortic regurgitation.
Although the VO2 and oxygen pulse curves showed significant LV systolic dysfunction during CPET, the hemodynamic parameters monitored (heart rate and blood pressure) presented normal behavior. These findings are of major significance, since a fall in blood pressure is a specific indicator of impaired ventricular function during exercise.8 However, the results obtained suggest that ventilatory variables were more sensitive than hemodynamic ones in identifying LV systolic dysfunction during exercise.
The stress Doppler echocardiogram confirmed the existence of systolic LV dysfunction during dynamic physical exercise, characterized by increased end-systolic volume accompanied by a marked fall in LV ejection fraction. These findings were clearly correlated with those observed during CPET.
CPET is not routinely applied in the context of valvular heart disease. It is more indicated to identify reduced exercise tolerance and to trigger symptoms in patients who are apparently asymptomatic despite the presence of valvular disease.5,8,9
Despite the scarcity of reports of this nature, in this patient with chronic AR CPET was able to demonstrate not only her low functional capacity, but also significant hemodynamic involvement due to valvulopathy during dynamic physical exercise.
It should be pointed out that chronic AR is a valvulopathy of slow and insidious evolution, and patients may remain asymptomatic for a long period of time.10,11 The complex pathophysiological mechanisms involved make it difficult to select the best therapeutic approach.5,12 For this reason, chronic AR is a challenge for cardiologists regarding the best time for surgery.
According to current guidelines, the following clinical conditions are considered to be class I evidence recommendations for valve surgery in severe chronic AR: in symptomatic patients or patients with LV ejection fraction lower than 50% at rest, or concomitantly with coronary artery bypass grafting or surgery on the aorta or other heart valves.5,12
After clinical discussion, considering the symptoms reported, the estimated severity of aortic regurgitation by cardiac MRI and the effects of CPET on ventilatory variables, it was decided to refer the patient for valve surgery, which was performed successfully.
Within this context, the judicious use of CPET is presented as a method for quantification of cardiac reserve that can aid in investigation and clinical management of patients with chronic AR.
FundingResearch supported by FAEPA HCFMRP-USP, FAPESP.
Conflicts of interestThe authors have no conflicts of interest to declare.









![Heart rate (bpm), VO2 (ml/min) and oxygen pulse (VO2/HR [ml/systole] responses. The vertical bars indicate the beginning and end of exercise.](https://static.elsevier.es/multimedia/08702551/0000003200000005/v1_201308021350/S0870255113000747/v1_201308021350/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)