Fragmented QRS complexes (fQRS) are a sign of myocardial scar and are associated with adverse outcomes and mortality in patients with coronary artery disease (CAD). However, little is known about the significance of fQRS or of their localization on electrocardiography (ECG) in patients without known CAD. We aimed to investigate the association between localization of fQRS on ECG and CAD severity in patients undergoing a first diagnostic coronary angiography.
MethodsWe enrolled 135 consecutive patients who had a narrow fQRS on ECG and underwent a first diagnostic coronary angiography. Patients were divided into two groups based on localization of fQRS on ECG (group 1: fQRS in the inferior leads, group 2: fQRS in the anterior leads). The groups were compared regarding the presence of significant CAD, multivessel disease (MVD) and SYNTAX score (SXscore).
ResultsEighty-six (63.7%) patients had fQRS in the inferior leads and 49 (36.3%) had fQRS in the anterior leads. There was no statistically significant difference between the groups regarding presence of significant CAD (47.7% vs. 51%, p=0.708). However, the incidence of MVD was significantly higher in group 2 compared to group 1 (10.5% vs. 28.6%, p=0.007). The median SXscore (6 [3,12] vs. 8 [4,24], p=0.047), and incidence of patients with SXscore >22 were significantly higher in group 2 compared to group 1 (3.5% vs. 20.4%, p=0.009).
ConclusionThe presence of fQRS in the anterior leads may indicate more severe CAD compared to fQRS in the inferior leads in patients undergoing a first diagnostic coronary angiography.
O QRS fragmentado (QRSf) é um sinal de cicatriz miocárdica e está associado a resultados adversos e a mortalidade em doentes com doença coronária (DC). No entanto, não existem dados claros sobre o significado de QRSf e sobre a localização do QRSf no eletrocardiograma (ECG) em doentes com DC conhecida. Este artigo tem o objetivo de investigar a associação entre a localização do QRSf no ECG e a gravidade da DC em doentes submetidos a um primeiro diagnóstico por angiografia coronária.
MétodosForam registados neste estudo 135 doentes consecutivos que apresentaram um QRSf estreito no ECG e foram submetidos a um primeiro diagnóstico por angiografia coronária. Os doentes foram divididos em dois grupos, com base na localização do QRSf no ECG (grupo 1: QRSf nas derivações inferiores; grupo 2: QRSf nas derivações anteriores). Foi feita a comparação entre os dois grupos atendendo à presença de DC significativa, doença multivasos (DMV) e score SYNTAX (score SX).
ResultadosOitenta e seis (63,7%) doentes apresentaram QRSf nas derivações inferiores e 49 (36,3%) doentes nas derivações anteriores. Não se registou diferença estatística significativa entre os dois grupos relativamente à presença de DC significativa (47,7 versus 51%, p=0,708). No entanto, a incidência de doentes com DMV foi significativamente superior no grupo 2, quando comparado com o grupo 1 (10,5 versus 28,6%, p=0,007). O score SX médio (6 [3,12] versus 8 [4,24], p=0,047) e a incidência de doentes com score SX > 22 foram significativamente superiores no grupo 2, quando comparado com o grupo 1 (3,5 versus 20,4%, p=0,009).
ConclusãoA presença de QRSf nas derivações anteriores pode estimular mais a DC grave, quando comparado com o QRSf nas derivações inferiores dos doentes submetidos a primeiro diagnóstico por angiografia coronária.
Coronary artery disease (CAD) is an important cause of morbidity and death. The diagnosis and assessment of CAD involves clinical evaluation, non-invasive tests and coronary angiography.1 Despite improvements in non-invasive diagnostic methods, stress tests have limited sensitivity and specificity to predict CAD severity in many cases.1–3 Hence, in addition to conventional risk factors, further risk stratifications may be useful to estimate CAD severity before coronary angiography. Fragmented QRS complexes (fQRS) are an electrocardiographic sign of heterogeneous ventricular conduction delay and are strongly correlated with myocardial fibrosis and ischemia.4 fQRS has been defined as the presence of notching in the R or S waves in the absence of typical bundle branch block or an additional wave such as RSR’ pattern in the QRS complex (<120 ms) in two contiguous leads in one of the major coronary artery territories.5 It is a predictor of adverse outcomes and mortality in patients with CAD, and CAD severity is a predictor of the presence of fQRS on electrocardiography (ECG).4–6 The presence of fQRS increases the positive predictive value of positive stress tests and is associated with a higher incidence of significant CAD in patients undergoing coronary angiography.7 However, little is known about the association between localization of fQRS on ECG and CAD severity in patients undergoing a first diagnostic coronary angiography.
The SYNTAX score (SXscore) is a comprehensive angiographic scoring system based on lesion characteristics and coronary anatomy. It is a useful tool to determine the complexity and severity of CAD, and is also associated with prognosis.8–12 In this study, we aimed to investigate the association between localization of fQRS on ECG and CAD severity in patients undergoing a first diagnostic coronary angiography.
MethodsAmong patients referred for a first diagnostic coronary angiography for suspected CAD, 135 consecutive patients who had a narrow fQRS in the inferior or anterior leads on ECG were enrolled in the study. Patients with fQRS in both inferior and anterior leads, acute coronary syndromes, left ventricular hypertrophy, ejection fraction <50%, previous history of CAD or vascular disease, complete or incomplete bundle branch block, QRS duration ≥120 ms or severe valvular heart disease were excluded at baseline. All patients underwent coronary angiography following positive exercise treadmill test or myocardial perfusion scintigraphy. Patients’ characteristics including ECG, laboratory measurements and cardiovascular risk factors were evaluated and recorded before coronary angiography. Hypertension was defined as blood pressure >140/90 mmHg or treatment with antihypertensive medication, and diabetes was defined as at least two fasting plasma glucose measurements ≥126 mg/dl and/or two-hour plasma glucose ≥200 mg/dl or HbA1c ≥6.5% or receiving antidiabetic drugs. Dyslipidemia was defined as fasting serum total cholesterol >200 mg/dl, low-density lipoprotein (LDL) cholesterol >160 mg/dl, serum triglyceride (TG) >180 mg/dl, or the use of lipid-lowering drugs because of a history of dyslipidemia. Documented myocardial infarction in first-degree male relatives aged <55 years or in first-degree female relatives aged <65 years was accepted as a family history of premature CAD. Active smoking was defined as the current regular use of cigarettes.
The study was approved by the local ethics committee and the study protocol complied with the Declaration of Helsinki.
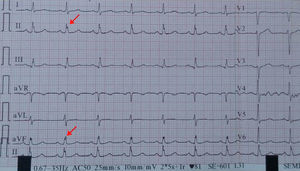
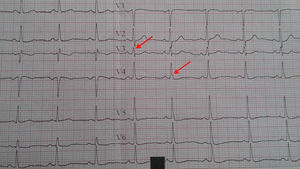
Electrocardiography and fQRSA 12-lead surface electrocardiogram was obtained from all patients on admission. fQRS was defined as the presence of notching in the R or S waves in the absence of typical bundle branch block or an additional wave such as RSR’ pattern in the QRS complex (<120 ms) in two contiguous leads in one of the major coronary artery territories5 (Figures 1 and 2).
The patients were divided into two groups before coronary angiography according to the presence of fQRS in the inferior (group 1) or anterior leads (group 2).
Angiographic analysis and coronary artery disease severityCoronary angiography was performed via a femoral or radial approach according to the standard Judkins technique. Coronary angiograms were evaluated by two experienced interventional cardiologists who were totally blinded to the study. Significant CAD was defined as ≥70% stenosis by quantitative angiography in ≥1 major coronary artery or ≥50% stenosis in the left main coronary artery. The presence of significant CAD, number of vessels with significant CAD, and SXscore were used to define CAD severity. Lesion severity was assessed in multiple projections in all patients. The SXscore was calculated for all coronary lesions with >50% stenosis in a >1.5 mm vessel, using the SXscore calculator (www.syntaxscore.com). An SXscore of >22 was defined as intermediate-high and ≤22 as low SXscore. All angiographic variables were assessed by two experienced interventional cardiologists who were blinded to the ECG findings and other procedural data. In cases of disagreement, the final decision was achieved by consensus.
The two groups were compared regarding presence of significant CAD, multivessel disease (MVD) and SXscore.
Statistical analysisStatistical analyses were performed using SPSS software version 15 (SPSS Inc., Chicago, Illinois). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov or Shapiro-Wilks tests) to determine whether they are normally distributed. Descriptive variables were presented using medians and interquartile range for non-normally distributed variables. Descriptive variables were presented using frequencies and proportions for ordinal variables. Since the continuous variables were not normally distributed, the Mann-Whitney U test was conducted to compare these parameters. The proportions of categorical variables were compared with the chi-square test or Fisher's exact test (when chi-square test assumptions did not hold due to low expected cell counts), where appropriate. A p-value of less than 0.05 was considered to show statistically significant results.
ResultsAmong 135 patients with fQRS, 86 (63.7%) had fQRS in the inferior leads (group 1) and 49 (36.3%) had fQRS in the anterior leads (group 2). The baseline demographic and laboratory characteristics of the two groups are presented in Table 1. There were no statistically significant differences between the groups concerning age, left ventricular ejection fraction, gender, hypertension, diabetes, dyslipidemia, cigarette smoking, family history or number of leads with fQRS. Coronary angiographic findings are shown in Table 2. There was no significant difference between the groups regarding presence of significant CAD (47.7% vs. 51%, p=0.708). However, the incidence of patients with MVD was significantly higher in group 2 than in group 1 (10.5% vs. 28.6%, p=0.007). Comparing the groups in terms of CAD severity according to SXscore, median SXscore was significantly higher in group 2 than group 1 (6 [3,12] vs. 8 [4,24], p=0.047). Additionally, the incidence of patients with SXscore >22 was significantly higher in group 2 (3.5% vs. 20.4%, p=0.009).
Baseline clinical and laboratory parameters of the two groups.
| fQRS in inferior leads (n=86) | fQRS in anterior leads (n=49) | p | |
|---|---|---|---|
| Age, years (median [IQR]) | 50 [46,54] | 50 [46.5,53] | 0.599a |
| LVEF, % (median [IQR]) | 62 [60,64] | 62 [60,64] | 0.655a |
| No. of leads (median [IQR]) | 2 [2,2] | 2 [2,2] | 0.865a |
| HGB, g/dl (median [IQR]) | 14.7 [13.9,15.3] | 14.6 [13.45,15.1] | 0.165a |
| Creatinine, mg/dl (median [IQR]) | 0.80 [0.67,0.90] | 0.80 [0.60,0.9] | 0.644a |
| WBC, 103/ml (median [IQR]) | 7.10 [6.80,7.95] | 7.20 [6.60-8.10] | 0.982a |
| LDL, mg/dl (median [IQR]) | 98 [78,122] | 101 [88.50,121] | 0.168a |
| TG, mg/dl (median [IQR]) | 122 [95.5,152] | 123 [99,144.5] | 0.674a |
| Gender | 0.931b | ||
| Male, n (%) | 52 (60.5) | 30 (61.2) | |
| Female, n (%) | 34 (39.5) | 19 (38.8) | |
| Hypertensive, n (%) | 28 (32.6) | 15 (30.6) | 0.815b |
| Diabetic, n (%) | 26 (30.2) | 15 (30.6) | 0.963b |
| Family history of CAD, n (%) | 26 (30.2) | 15 (30.6) | 0.963b |
| Smokers, n (%) | 26 (30.2) | 16 (32.7) | 0.770b |
CAD: coronary artery disease; HGB: hemoglobin; IQR: interquartile range; LDL: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; TG: triglycerides; WBC: white blood cell count.
Comparison of angiographic findings in the two groups.
| fQRS in inferior leads (n=86) | fQRS in anterior leads (n=49) | p | |
|---|---|---|---|
| Significant CAD, n (%) | 41 (47.7) | 25 (51) | 0.708a |
| Multivessel CAD, n (%) | 9 (10.5) | 14 (28.6) | 0.007a |
| SXscore >22, n (%) | 3 (3.5) | 10 (20.4) | 0.009b |
| SXscore (median [IQR]) | 6 [3,12] | 8 [4,24] | 0.047c |
CAD: coronary artery disease; IQR: interquartile range; SXscore: SYNTAX score.
The main finding of the present study is that the presence of fQRS in the anterior leads is associated with a higher incidence of MVD and higher SXscore levels compared to fQRS in the inferior leads in patients without known CAD or vascular disease undergoing a first diagnostic coronary angiography. This finding suggests that fQRS in the anterior leads may indicate more severe CAD than fQRS in the inferior leads. The presence of fQRS on ECG is a sign of myocardial fibrosis and scar and is associated with adverse outcomes and increased mortality in patients with CAD.4,13 Additionally, it has been shown that the presence of fQRS on ECG is associated with a higher incidence of significant CAD compared to absence of fQRS in patients with chest pain considered to have an intermediate pretest likelihood of significant CAD and referred for diagnostic coronary angiography based on positive stress test.7 However, little is known about the association between localization of fQRS and CAD severity in patients referred for diagnostic coronary angiography. In a population-based study, Terho et al.14 demonstrated that the presence of fQRS on ECG is not an uncommon finding and reported that fQRS in both inferior and anterior leads was not associated with increased risk of mortality in subjects without known cardiac disease. Hence, the importance of fQRS and localization of fQRS in patients without known cardiovascular disease remains unclear. Our findings reveal that fQRS in the anterior leads is associated with more severe CAD than fQRS in the inferior leads, defined by MVD and SXscore, in patients undergoing a first diagnostic coronary angiography.
Although the best modality to determine CAD severity, elective coronary angiography has a low diagnostic yield.15 Only a third of patients without known disease who undergo elective coronary angiography have a diagnosis of obstructive CAD.15,16 Therefore, better strategies for risk stratification are needed to inform decisions and to increase the diagnostic yield of coronary angiography. In our study, 47.7% of patients in group 1 and 51% of patients in group 2 had a diagnosis of obstructive CAD. Hence, the presence of fQRS seems to increase the diagnostic yield of coronary angiography. Also, we found that fQRS in the anterior leads are associated with more severe CAD than fQRS in the inferior leads. These findings also suggest that the presence of fQRS on ECG and localization of fQRS may be used for risk stratification in patients undergoing elective coronary angiography.
Our study has several limitations. First, the study population was relatively small. However, we were still able to demonstrate a significant association between localization of fQRS on ECG and CAD severity. Second, there is a lack of data about coronary hemodynamics and microvascular dysfunction that may play a role in myocardial ischemia in patients who have a positive stress test.
ConclusionfQRS is a simple and accessible non-invasive parameter that can be easily identified by routine ECG. Beyond its prognostic value in patients with CAD, it may be used to improve the diagnostic yield of elective coronary angiography in patients without known CAD or vascular disease. In particular, the presence of fQRS in the anterior leads may be used as an important marker of more severe CAD in patients undergoing a first diagnostic angiography.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.