Randomized controlled trials have yielded conflicting results regarding the impact of beta-blockers on perioperative cardiovascular morbidity and mortality. This Cochrane systematic review assessed the impact of this intervention on mortality and cardiovascular events.
Eighty-eight randomized controlled trials with 19 161 participants were included (53 trials on cardiac surgery and 35 trials on non-cardiac surgery).
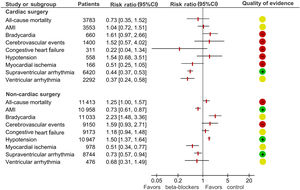
In cardiac surgery perioperative beta-blockers had a protective effect against supraventricular and ventricular arrhythmias but had no significant effect on mortality or on the occurrence of acute myocardial infarction (AMI), stroke, heart failure, hypotension or bradycardia. In non-cardiac surgery, beta-blockers had a protective effect against AMI and arrhythmias, but this was counterbalanced by an increased risk of death and stroke.
In conclusion, perioperative use of beta-blockers appears overall to be beneficial in cardiac surgery. However, in non-cardiac surgery the substantial reduction in rhythm disturbances and AMI appears to be offset by an increase in mortality and stroke, and so the systematic use of beta-blockers in this setting is not recommended.
Os resultados de ensaios clínicos aleatorizados relativos à utilização de betabloqueantes no período perioperatório de cirurgia cardíaca e não cardíaca têm sido controversos. Esta revisão sistemática da Cochrane avaliou o impacto dessa intervenção na mortalidade e eventos cardiovasculares peri-operatórios. Foram incluídos 88 ensaios clínicos aleatorizados com um total de 19 161 participantes (53 ensaios com cirurgia cardíaca e 35 com cirurgia não cardíaca).
A utilização perioperatória de betabloqueantes na cirurgia cardíaca reduziu significativamente a ocorrência de disritmias supraventriculares e ventriculares, sem impacto significativo na mortalidade, bem como no risco de enfarte agudo do miocárdio (EAM), acidente vascular cerebral (AVC), insuficiência cardíaca, hipotensão e bradicardia. Por outro lado, na cirurgia não cardíaca, apesar de existir uma redução no risco de EAM e nos eventos arrítmicos supraventriculares, esse efeito foi contrabalançado pelo aumento do risco de morte e AVC.
Em conclusão, a utilização de betabloqueantes no período perioperatório de cirurgia cardíaca parece ser benéfica. Por outro lado, na cirurgia não cardíaca apesar da redução significativa de EAM e disritmias, há um aumento da mortalidade e de AVC, pelo que a utilização sistemática de betabloqueantes em doentes submetidos a cirurgia não cardíaca não está recomendada.
What is the impact of beta-blockers on perioperative adverse events?1
Description of reviewThis is a systematic review of randomized controlled trials (RCTs) on the effects of perioperatively administered beta-blockers for prevention of surgery-related mortality and morbidity in patients undergoing any type of surgery while under general anesthesia.
The following outcomes were assessed: all-cause mortality; acute myocardial infarction (AMI); myocardial ischemia; cerebrovascular events; hypotension; bradycardia; congestive heart failure; ventricular arrhythmias; supraventricular arrhythmias; length of hospital stay.
ResultsThe authors performed a comprehensive database analysis for trials fulfilling the inclusion criteria (Cochrane Central Register of Controlled Trials [CENTRAL], MEDLINE, EMBASE, Biosis Previews, CAB Abstracts, Cumulative Index to Nursing and Allied Health Literature [CINAHL], Derwent Drug File, Science Citation Index Expanded, Life Sciences Collection, Global Health and PASCAL).
Eighty-eight RCTs with 19 161 participants were included (53 trials on cardiac surgery and 35 trials on non-cardiac surgery). Outcomes were assessed separately for cardiac and non-cardiac surgery.
Regarding cardiac surgery, beta-blockers had a protective effect against supraventricular and ventricular arrhythmias. There was no evidence of an effect on death, the occurrence of AMI, stroke or heart failure, or the development of disproportionately low blood pressure or bradycardia during surgery. Length of hospital stay after heart surgery was reduced by about 0.5 days in patients taking beta-blockers (Figure 1).
Plot showing the results of the meta-analysis assessing the impact of beta-blockade on the outcomes of patients undergoing cardiac and non-cardiac surgery. The quality of evidence as reported in the review1: low quality (red), moderate quality (yellow), and high quality (green). AMI: acute myocardial infarction; CI: confidence interval.
In non-cardiac surgery, beta-blockers increased the risk of death and stroke, the latter only when a representative group of high-quality trials was analyzed. The protective effect against AMI and rhythm disturbances was counterbalanced by this increased risk of death and stroke (Figure 1).
ConclusionsIn conclusion, perioperative use of beta-blockers appears beneficial overall in cardiac surgery, particularly concerning the risk of ventricular and supraventricular arrhythmias. In non-cardiac surgery, the evidence shows an increase in death and a potential increase in stroke with the use of these drugs. The substantial reduction in rhythm disturbances and AMI in this setting appears to be offset by this potential increase in mortality and stroke.
CommentsThis systematic review presents the best available evidence on the controversial topic of the perioperative use of beta-blockers in cardiac or non-cardiac surgery.1 Perioperative cardiovascular complications are an important concern because 2% of patients suffer major cardiac complications,2 and 8% show evidence of significant myocardial injury.3
The perioperative use of beta-blockers was initially seen as a potential way to decrease the risk of perioperative cardiovascular complications, particularly after the publication of two RCTs with positive results.4,5 On the basis of this evidence, the first versions of the clinical practice guidelines recommended the use of beta-blockers in the perioperative period in patients undergoing non-cardiac surgery. Nonetheless, for various reasons, the strength and scope of these recommendations have decreased in later versions of the guidelines. First, the benefits of perioperative beta-blockers could not be reproduced in other RCTs. Second, the POISE-1 trial, which enrolled 9000 participants, showed that the use of beta-blockers decreased the risk of perioperative AMI but increased the risks of death, stroke, bradycardia and hypotension.6 These adverse events were likely related to one of the major criticisms of the POISE-1 trial, namely starting long-acting beta blockers at high doses shortly prior to surgery.6 Third, the validity of work led by the Dutch investigator Don Poldermans has come under scrutiny, and two RCTs (the DECREASE trials) with positive results have been retracted due to concerns about scientific misconduct.5,7 Consequently, the guidelines re-evaluated these data and excluded them from the background evidence used to set recommendations for perioperative beta-blockade.
As an example, the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines supported their recommendations by their own systematic review on this topic, from which they excluded the two DECREASE trials. The review identified 16 RCTs including 12 043 participants and the pooled results showed that prophylactic beta-blocker use resulted in 17 fewer cases of AMI, at the cost of four excess strokes and six deaths for every 1000 patients.8 Nevertheless, the review identified major limitations: no valid trials assessed beta-blockade that was started more than 24 hours before surgery, and very few trials assessed agents aside from metoprolol. Therefore, the ACC/AHA guidelines advise against initiating beta-blocker therapy in the 24 hours before surgery. Based on observational studies, starting beta-blocker therapy might be considered in patients at high cardiovascular risk or with reversible ischemia detected on stress testing.8
Importantly, the above discussion is mostly relevant to patients beginning beta-blockers de novo, and does not apply to patients previously treated with beta-blockers. As withdrawal of chronic therapy is associated with an increase in the risk of cardiovascular events and death, beta-blocker therapy should be continued, although modification of perioperative doses or discontinuation may be required to address changing clinical circumstances such as hypotension, bradycardia, or massive blood loss.
The current European Society of Cardiology guidelines on non-cardiac surgery only recommend beta-blocker therapy (preferably with atenolol or bisoprolol based on observational studies)9-11 in high-risk patients undergoing high-risk surgery.12 Based on clinical judgment and evidence from small trials, beta-blockers should be initiated and titrated in order to achieve a heart rate between 60 and 70 bpm and to avoid a systolic blood pressure below 100 mmHg.13,14
The clinical question posed here clearly needs to be answered with new randomized controlled trials to determine (1) which patients derive benefit from beta-blocker therapy in the perioperative setting; (2) if there is a class effect or whether there is a single best beta-blocker (if any) for this setting; (3) what is the best time to start beta-blockers, or what is the period of greatest risk; and (4) what are the optimum doses and/or hemodynamic targets (including blood pressure and heart rate) for beta-blocker therapy.
In cardiac surgery, there are many situations in which beta-blockers are already indicated. However, in addition, beta-blockers may be useful to decrease the risk of supraventricular (including atrial fibrillation) and ventricular arrhythmias that may result from increased sympathetic tone following surgery.
Conflicts of interestThe authors have no conflicts of interest to declare.