Cardiac dysfunction among cirrhotic patients has long been recognized in the medical community. While it was originally believed to be a direct result of alcohol toxicity, in the last 30 years cirrhotic cardiomyopathy (CCM) has been described as a syndrome characterized by chronic cardiac dysfunction in cirrhotic patients in the absence of known cardiac disease, regardless of the etiology of cirrhosis. CCM occurs in about 60% of patients with cirrhosis and plays a critical role in disease progression and treatment outcomes. Due to its predominantly asymptomatic course, diagnosing CCM is challenging and requires a high index of suspicion and a multiparametric approach. Patients with CCM usually present with the following triad: impaired myocardial contractile response to exercise, inadequate ventricular relaxation, and electrophysiological abnormalities (notably prolonged QT interval). In recent years, research in this area has grown expeditiously and a new set of diagnostic criteria has been developed by the Cirrhotic Cardiomyopathy Consortium, to properly identify patients with CCM. Nevertheless, CCM is still largely unknown among clinicians, and a major part of its pathophysiology and treatment is yet to be understood. In the present work, we aim to compile and summarize the available data on the pathogenesis, clinical features, diagnosis, treatment, and prognosis of CCM.
A disfunção cardíaca observada em doentes cirróticos é reconhecida há muito tempo pela comunidade médica. Embora originalmente se acreditasse ser o resultado direto da toxicidade pelo álcool, nos últimos trinta anos a cardiomiopatia cirrótica (CCM) foi descrita como uma síndrome de disfunção cardíaca em doentes cirróticos na ausência de doença cardíaca conhecida, independentemente da etiologia da cirrose. A CCM está presente em até 60% dos doentes com cirrose e desempenha um papel crítico na progressão da doença e no resultado do tratamento. Devido ao seu curso predominantemente assintomático, o diagnóstico de CCM é desafiante e requer um elevado índice de suspeição e uma abordagem multiparamétrica. Doentes com CCM geralmente apresentam a seguinte tríade: resposta contrátil do miocárdio ao exercício anormal, perturbação do relaxamento ventricular e anomalias eletrofisiológicas (nomeadamente, prolongamento do intervalo QT). Nos últimos anos, a pesquisa nesta área aumentou rapidamente e um novo conjunto de critérios diagnósticos foi desenvolvido pelo Consórcio da Cardiomiopatia Cirrótica, para melhor identificar estes doentes. No entanto, este tema é ainda amplamente desconhecido por muitos clínicos e a grande parte da sua fisiopatologia e tratamento estão ainda por esclarecer. Nesta revisão, o nosso objetivo é compilar e sumariar a informação disponível sobre a patogénese, apresentação clínica, diagnóstico, tratamento e prognóstico da CCM.
The relationship between the liver and the heart has been well established over the years. The progressively reduced systemic vascular resistance (SVR) seen in cirrhotic patients causes pronounced arterial vasodilatation, which leads to hemodynamic changes.1 These changes lead to a hyperdynamic circulation, characterized by increased cardiac output (CO) and raised heart rate (HR).1–4 In cirrhotic patients, it is not unusual for other conditions to coexist that can cause or contribute to cardiac dysfunction, such as hypertension, ischemic heart disease, anemia and excessive alcohol consumption. The relationship between cirrhosis and cardiac disease is thus not clear, and for many years it was believed that the cardiac impairment seen in cirrhotic patients was a direct consequence of the cardiotoxic effect of alcohol.3,5,6 The term ‘cirrhotic cardiomyopathy’ (CCM), coined by Lee7 more than three decades ago, is defined as chronic cardiac dysfunction in cirrhotic patients in the absence of known cardiac disease. These cardiac abnormalities appear to be independent of the etiology of the cirrhosis.7–9
The prevalence of CCM remains unclear, but it is believed to be currently underdiagnosed, since it is usually latent and tends to manifest when patients are under stress.10,11 A study by Razpotnik et al. that included 122 patients with cirrhosis without other causes of structural heart disease found that CCM was present in around 60% of these patients.12 In a prospective study carried out by Anikhindi et al., 50% of the 104 cirrhotic patients included were diagnosed with CCM.8 Evidence regarding the predictors of CCM is still sparse. In Anikhindi et al.’s study, age was found to be the strongest indicator for diastolic dysfunction (DD).8
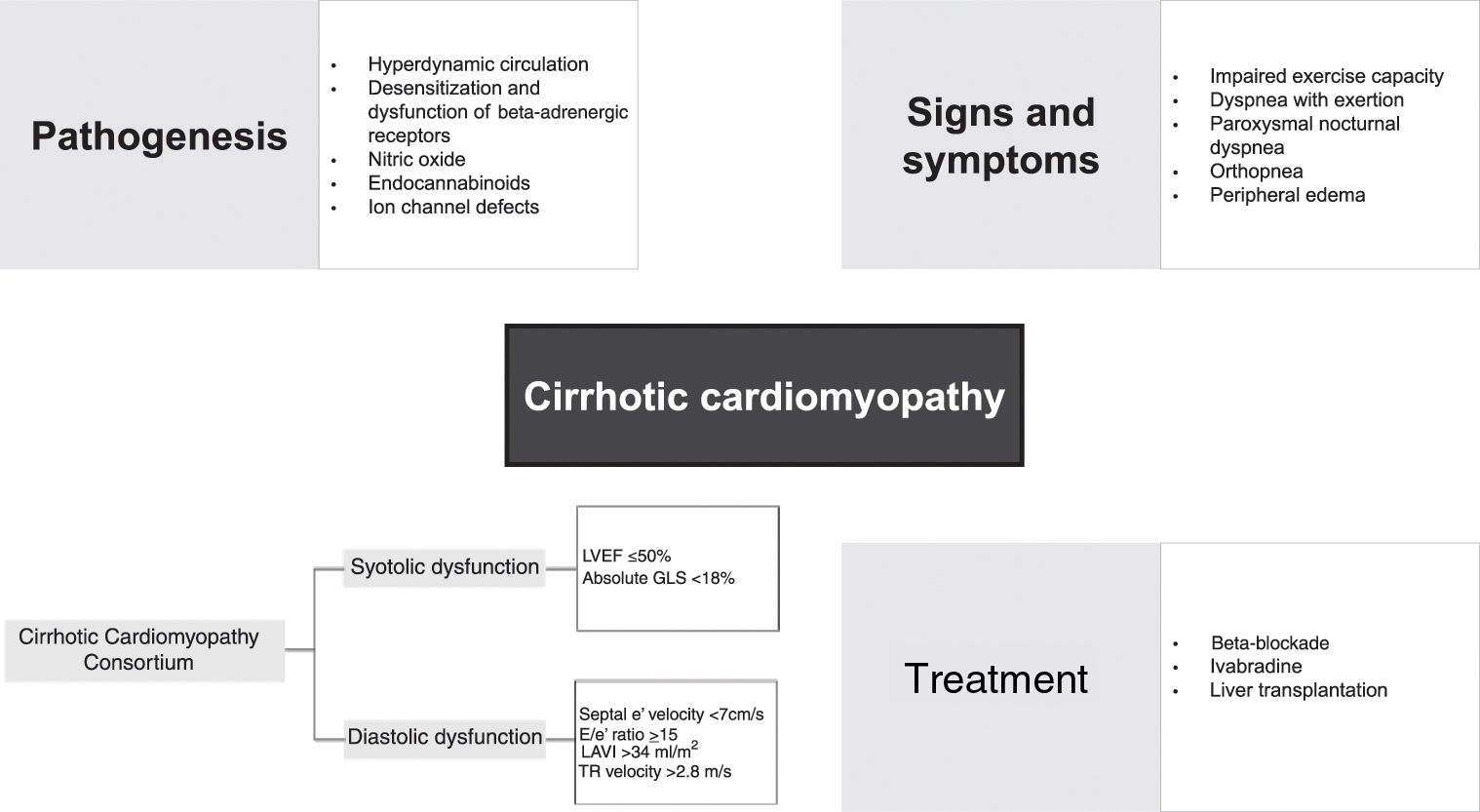
Although this entity is mainly subclinical, it manifests in the context of hemodynamic stress, such as exercise, use of certain drugs, liver transplantation (LTx) and transjugular intrahepatic portosystemic shunt (TIPS) insertion.2,13–15 A study by Busk et al. with a group of 25 cirrhotic patients referred for TIPS insertion found that this procedure caused rapid increases in preload and central blood volume, and that these changes resulted in impaired diastolic, systolic, and electrophysiological function.16 Ginès et al. investigated the effect of TIPS versus large-volume paracentesis for treatment of ascites and reported an increased risk of developing heart failure (HF) following TIPS; HF was present in 12% of the TIPS group and was not seen in the paracentesis group.17 When HF is found, it may present with symptoms such as dyspnea with exertion, impaired exercise capacity, paroxysmal nocturnal dyspnea, peripheral edema, and orthopnea.18
CCM is associated with other complications of cirrhosis, including ascites, hepatic encephalopathy and esophageal varices, and treating these complications does not modify the natural history of CCM.14 CCM may play an important role in the pathogenesis of hepatorenal syndrome (HRS) precipitated by spontaneous bacterial peritonitis (SBP). Studies by Ruiz-del-Arbol et al. in patients recovering from SBP revealed that HRS occurred in those whose CO was lower at baseline and failed to increase after infection resolution, and that a lower CO was predictive of HRS occurrence.19,20
The aim of this review is to discuss various pathophysiologic mechanisms, highlight the current diagnostic criteria and areas of conflicting evidence, and summarize the current available treatment options.
MethodsArticle research was conducted using the search term “Cirrhotic cardiomyopathy” in the PubMed database. We limited our research to papers written in English and Portuguese between 2000 and 2023. This produced 395 articles, of which, after abstract screening, 129 were deemed to be of possible relevance. Of these, 70 were included in the present work. Additional research on certain areas was performed when deemed relevant and all references were included.
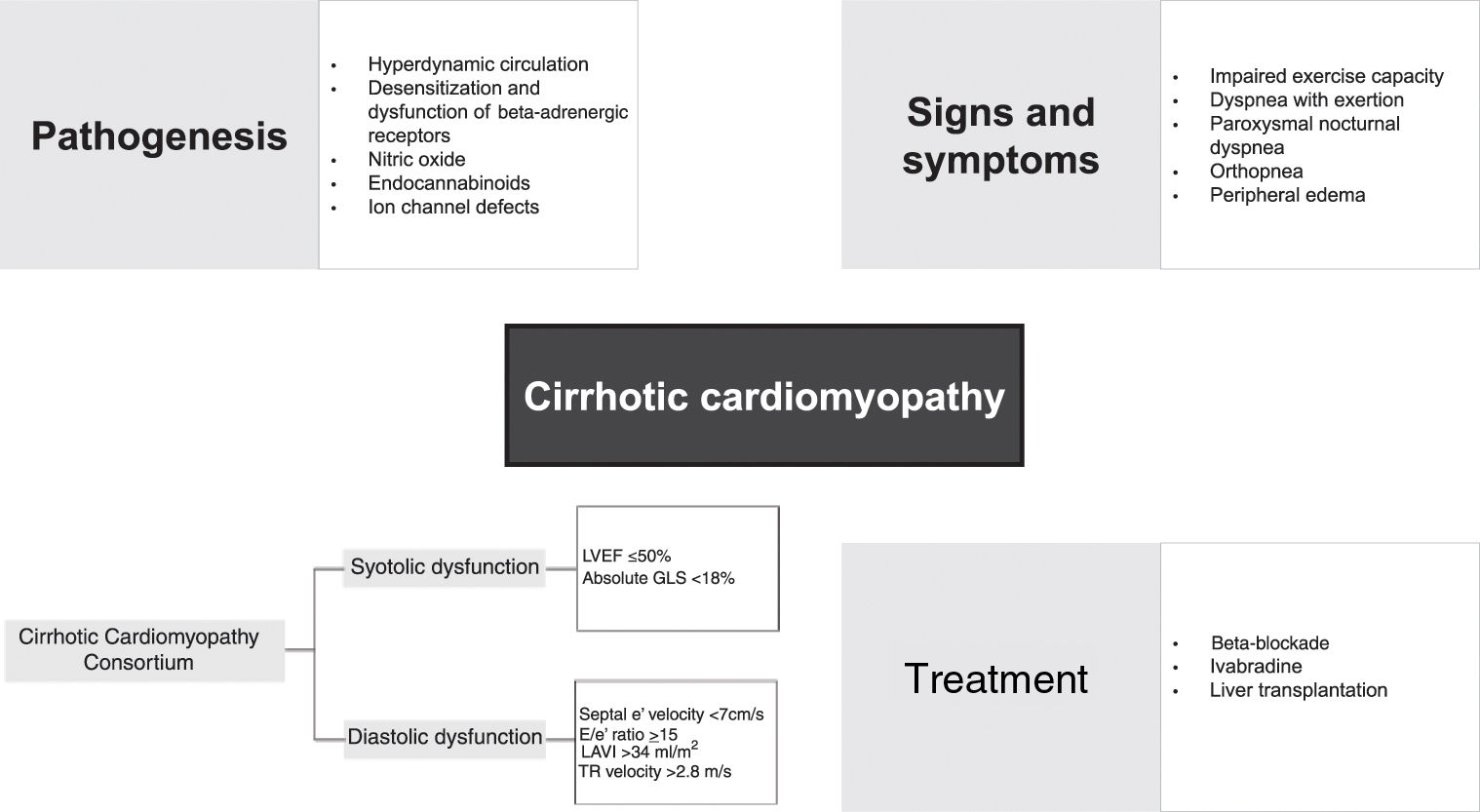
PathogenesisThe mechanisms involved in the pathophysiology of CCM are complex and involve various cellular and neurohumoral pathways, such as impaired beta-receptor signaling, sympathetic nervous system activation, and activity of many vasoactive substances, including nitric oxide (NO) and endocannabinoids.
Hyperdynamic circulationThe hyperdynamic circulatory state found in cirrhosis is defined by an increase in total blood volume, a reduction in circulating volume due to splanchnic blood pooling, reduced SVR and increased CO.21 Reduced effective arterial volume leads to the activation of central baroreceptors, the sympathetic nervous system, the renin–angiotensin–aldosterone system (RAAS) and the arginine–vasopressin system, leading to increases in HR and preload due to salt and fluid retention.4,10 This hyperdynamic state with a normal-high CO can mask signs and symptoms of HF during the progression of the disease.1 With disease progression, SVR decreases further and even more blood accumulates in the splanchnic circulation, leading to progressively reduced central blood volume.1 A vicious cycle thus begins, with high CO unable to compensate for low SVR.1
Beta-adrenergic responsivenessBeta-adrenergic responsiveness plays an important role in cardiac muscle contractility and chronotropy.3 A study by Lee et al. using a rat model of biliary cirrhosis found a significantly lower density of beta-adrenergic receptors in cirrhotic rats, associated with a decreased response to isoprenaline stimulation compared to control rats.22 These results support the theory that the prolonged vasodilation observed in cirrhotic patients leads to constant activation of the sympathetic system with desensitization and dysfunction of beta-adrenergic receptors2; beta-adrenergic receptor dysfunction is found in virtually all CCM patients.3 Curiously, another study also found that blunted muscarinic responsiveness is present in CCM, although this is most likely a compensatory mechanism and does not play an integral part in the genesis of CCM.23 Another explanation for impaired beta-adrenergic responsiveness in CCM is related to the altered lipid concentration of cell membranes.24 A significantly higher level of membrane cholesterol was found in a group of cirrhotic rats, with an increased cholesterol:phospholipid ratio, which led to reduced fluidity in the cardiac plasma membrane and impaired beta-adrenergic function.24 In rats with experimentally induced cirrhosis, abnormal gene expression related to the beta-adrenergic system at a post-receptor level led to down-regulation of this pathway and was associated with the early pathogenesis of CCM, especially overexpression of G-protein subunit alpha i2, cyclic nucleotide phosphodiesterase and regulator of G-protein signaling 2, and down-expression of adenylate cyclase.25
Nitric oxideNO is recognized as the key humoral mediator implicated in the etiology of hyperdynamic circulation.3 NO levels in cirrhotic patients are permanently elevated due to the response to transient bacteremia and increased endotoxins and cytokines.26 In the context of CCM, it is theorized that inducible NO synthase diminishes ventricular contractility.26 In 2000, an animal study found that significantly higher amounts of tumor necrosis factor-alpha (TNF-α), cyclic guanosine monophosphate (cGMP) and NO were present in cardiac homogenates obtained from cirrhotic rats, indicating a possible TNF-α-NO-cGMP mediated pathway in the pathogenesis of CCM.27 In the same study, the authors report reduced muscle contractile force compared with the control group, which increased significantly when the papillary muscles were incubated with a non-specific NO synthase inhibitor.27 Stimulation of NO synthase may thus play a major role in the pathogenesis of CCM.27
Like NO, carbon monoxide activates soluble guanylate cyclase, leading to increased levels of cGMP, which in turn depresses ventricular contractility by inhibiting intracellular calcium fluxes.28 Liu et al. found that messenger ribonucleic acid, protein expression of inducible heme oxygenase 1 (which degrades heme to biliverdin and carbon monoxide), and cGMP levels were significantly increased in the left ventricles of cirrhotic rats, and treatment with zinc protoporphyrin IX, a heme oxygenase inhibitor, led to normalized cGMP levels and restored ventricular papillary muscle contractility.29 These results indicate that carbon monoxide plays a role in the pathogenesis of CCM.
EndocannabinoidsMyocardial endocannabinoid production may be increased in response to increased HR and hemodynamic overload, both of which are seen in cirrhotic patients.30,31 Binding of endocannabinoids to cannabinoid receptor type 1 (CB1) receptors leads to a negative inotropic effect and hypotensive response in cirrhotic rat models.30,32,33 A study by Yang et al. on bile duct ligated mice showed that synthesis of the endocannabinoid anandamide led to depressed cardiac contractility and that administration of a CB1 antagonist improved contractility.34
CB1 antagonism has also been shown to normalize the blunted cardiac response to hemorrhage in cirrhotic rats and may reverse the ionotropic inhibitory effect of endocannabinoids, as well as improving peripheral vascular resistance and arterial blood pressure.35,36
Ion channel defectsIon flux, especially of sodium, potassium and calcium, plays a major role in cardiac contractility. While calcium is essential to maintain the plateau of the action potential, potassium and sodium are required in the generation of action potentials. Ward et al. reported that decreased cardiac contractility in cirrhotic cardiomyocytes is caused by dysfunction of the calcium regulatory system, with plasma membrane calcium channels quantitatively reduced and functionally depressed, resulting in a significant reduction in the peak of inward calcium current.37 Ion channel changes contribute to the impaired cardiac contractility and electrophysiological abnormalities seen in CCM, including corrected QT (QTc) interval prolongation.4,28,37
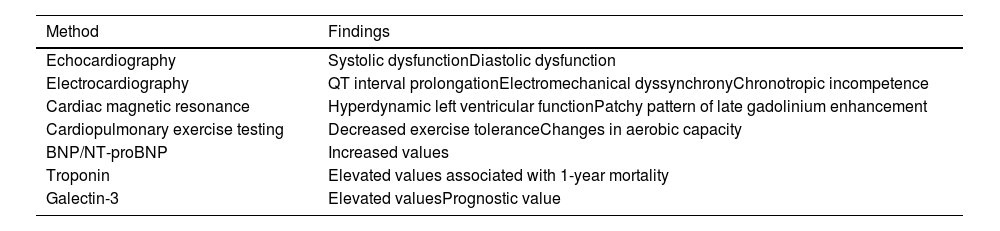
Clinical features and diagnosisPatients with CCM are usually asymptomatic at rest and myocardial impairment can be difficult to document on routine examination.2,10,12Table 1 presents possible diagnostic investigation of CCM. A high index of suspicion is required to make this diagnosis, which should be considered in all cirrhotic patients who present with signs and symptoms of heart failure or in whom an invasive technique, such as TIPS or LTx, is planned.
Diagnostic investigation of cirrhotic cardiomyopathy.
| Method | Findings |
|---|---|
| Echocardiography | Systolic dysfunctionDiastolic dysfunction |
| Electrocardiography | QT interval prolongationElectromechanical dyssynchronyChronotropic incompetence |
| Cardiac magnetic resonance | Hyperdynamic left ventricular functionPatchy pattern of late gadolinium enhancement |
| Cardiopulmonary exercise testing | Decreased exercise toleranceChanges in aerobic capacity |
| BNP/NT-proBNP | Increased values |
| Troponin | Elevated values associated with 1-year mortality |
| Galectin-3 | Elevated valuesPrognostic value |
BNP: B-type natriuretic peptide; NT-proBNP: N-terminal B-type natriuretic peptide.
The definition and diagnostic criteria of CCM are thus still evolving. At the 2005 Montreal World Congress of Gastroenterology, initial diagnostic criteria were proposed by an expert consensus panel. These criteria focused mainly on indices of systolic dysfunction, with a blunted contractile response on stress testing and left ventricular (LV) ejection fraction (LVEF) <55%, as well as signs of DD, such as prolonged mitral deceleration time (>200 ms), prolonged isovolumic relaxation time (>80 ms) and an age-corrected early to late diastolic filling ratio <1.0.38 Supportive criteria included electrophysiological abnormalities, abnormal chronotropic response, electromechanical uncoupling, prolonged QTc interval, enlarged left atrium, increased myocardial mass, and increased B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) or troponin I.38
Over the following years, with the development of imaging tests such as tissue Doppler imaging (TDI) and speckle-tracking echocardiography, allied with advances in understanding of HF, these criteria quickly became outdated.
In their systematic review and meta-analysis of 12 publications, Shahvaran et al. reported that using standard two-dimensional (2D) echocardiography leads to overdiagnosis of CCM in comparison to TDI, with a pooled prevalence of CCM of 61% using the former method falling to 45% with more recent techniques like TDI.39 This finding is supported by another study that compared diagnostic criteria using more recent techniques such as TDI and the 2005 Montreal criteria, with the Montreal criteria leading to overdiagnosis of patients with DD.12 Overdiagnosis of CCM following standard 2D echocardiography is thought to be due to the insensitivity of this technique in detecting DD and to inappropriate cut-off values, particularly the ratio of peak velocity of early diastolic transmitral flow to peak velocity of late transmitral flow (E/A).39 The cut-off of E/A <1 used in a healthy population results in an increase in false positives for the population-specific lowered baseline E/A that occurs in cirrhosis.39
In 2018, a group of multidisciplinary experts in the fields of hepatology, anesthesia and cardiology formed the Cirrhotic Cardiomyopathy Consortium and proposed new diagnostic criteria for the disease. According to these criteria, the diagnosis of CCM should be made based on the presence of systolic dysfunction, with LVEF ≤50% or global longitudinal strain (GLS) higher than −18% (smaller absolute value) in cirrhotic patients with preserved LVEF, associated with advanced DD revealed by the presence of at least three of the following: septal e′ velocity <7 cm/s, ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity (E/e′) ≤15, left atrial volume index >34 ml/m2, and tricuspid regurgitation (TR) velocity >2.8 m/s.40
In many cases, DD precedes systolic dysfunction, which tends to manifest only under conditions presenting as a cardiovascular challenge.
Other tests may be useful for diagnosing CCM and stratifying risk in these patients. Cardiopulmonary exercise testing may reveal some level of cardiovascular limitation in patients with CCM who are asymptomatic at rest and help to identify patients with end-stage liver disease who present with some exercise limitation due to other mechanisms such as abnormal pulmonary gas exchange or skeletal muscle metabolism, or deconditioning.40
ElectrocardiogramSeveral electrophysiological abnormalities are found in cirrhotic patients, the three most often reported being QT interval prolongation, electromechanical dyssynchrony and chronotropic incompetence.10
QT interval correction in patients with cirrhosis should be performed using the Fridericia formula, since less rigorous methods lead to an overestimation of QT prolongation.41–43 QT interval prolongation is present in up to 60% of cirrhotic patients and appears to increase in parallel with the severity of cirrhosis as assessed by the Child-Pugh score.42 However, it remains unclear whether this predisposes patients to more serious types of arrhythmias, and drugs known to prolong QT interval, such as quinolones or vasopressin analogues, should be used with caution in these patients.42,44 A retrospective study by Sonny et al. revealed that a longer QTc was independently associated with adverse long-term outcome after orthotopic LTx.44
A single-center study found that QTc interval was longer in patients with alcoholic etiology of cirrhosis than in those with viral hepatitis or other etiologies, as well as in patients with ascites and encephalopathy.43 These results show that QTc interval may be associated with the etiology and complications of cirrhosis.43 Interestingly, a retrospective cohort study including consecutive patients undergoing LTx between 2010 and 2018 showed that QT interval prolongation was not associated with the structural or functional cardiac abnormalities that characterize CCM, suggesting that QT prolongation in cirrhotic patients and CCM may be two distinct entities with distinct pathophysiological origins.45 There are some data suggesting a relationship between prolonged QT interval and increased plasma concentrations of endothelin-1, TNF-α and norepinephrine, autonomic dysfunction, structural myocyte abnormalities, and ion imbalance due to dysfunction of calcium and potassium channels.28,43
In patients with QT interval prolongation, the normally tightly coupled interval between the onset of the QRS complex on the electrocardiogram and LV contraction varies widely, a phenomenon known as electromechanical dyssynchrony.46
The clinical relevance of this finding remains unknown. Chronotropic incompetence is related to the inability of the heart to sufficiently raise HR in response to exercise or pharmacological stimulation.3 This finding has some prognostic relevance, as it is associated with an increased risk of perioperative complications, especially in patients undergoing LTx.3,47
Cardiac magnetic resonanceCardiac magnetic resonance (CMR) imaging is the optimal choice to accurately assess LVEF, chamber volumes, myocardial fibrosis and edema, which can be detected even prior to the onset of LV systolic dysfunction.48 Lossnitzer et al. found that CMR in patients with end-stage liver disease demonstrated hyperdynamic LV function with a patchy pattern of late gadolinium enhancement, with an appearance mimicking myocarditis.49 Indeed, these patients presented elevated NT-proBNP and high-sensitivity troponin T, indicating a concealed form of heart failure.49
In a prospective study by Isaak et al., myocardial T1 and T2 relaxation times were increased in participants with liver cirrhosis compared with healthy controls, and these parameters, as well as extracellular volume and the presence of late gadolinium enhancement, correlated with a higher Child-Pugh class, suggesting an association between clinical grade of liver disease and cardiac fibrosis and inflammation.50
Although it may come to play an important part in CCM diagnosis, the applicability of CMR is still low, mainly due to non-specific distribution patterns.48
Serum biomarkersPlasma concentrations of BNP and NT-proBNP, which are secreted by the ventricles under stress during diastole, are related to cardiac function and disease prognosis.2 In a study by Metwaly et al., BNP levels were significantly increased in cirrhotic patients compared to healthy controls and to patients with nonalcoholic fatty liver disease, and were correlated with the severity of liver disease.51 BNP levels were also increased in decompensated cirrhotic patients compared to those without decompensation, history of hepatic encephalopathy, variceal bleeding or SBP.51 Post-LTx, higher BNP is associated with increased risk of 30-day mortality and worse outcomes.52 This result is supported by Saner et al., who reported significantly higher Model for End-Stage Liver Disease score, dialysis treatment and mortality in a group of adult liver recipients with BNP >391 pg/ml compared to those who did not reach this level.53 BNP is an independent predictor of medium-term survival in cirrhosis; routine monitoring of peri-LTx BNP provides prognostic information and could assist in risk stratification for mortality as a pragmatic and useful biomarker.52–54
Troponin plays an important part in muscle contraction, and serum troponin I and troponin T are the most sensitive and specific biomarkers of cardiomyocyte damage.2 A retrospective analysis assessing the association between cardiac troponin T and one-year mortality in cirrhotic patients without known cardiovascular disease admitted to the emergency room reported increased cardiac troponin T levels in up to 49% of patients and showed that cardiac troponin T levels were independently associated with one-year mortality.55 Moon et al. analyzed a group of 2490 adult cirrhotic patients and demonstrated that combined elevation of pre-LTx BNP and high-sensitivity troponin I (hs-TnI) posed a higher risk of 90-day mortality after transplantation.56 Although this result is promising, whether BNP or hs-TnI has superior predictive value needs further investigation.56
Galectin-3 is a beta-galactoside-binding lectin that is significantly increased in cirrhotic patients and in animal models of liver fibrosis, and is associated with cardiac diastolic and systolic function.2,57,58 A case–control study with 71 patients reported that BNP and galectin-3 had higher sensitivity and specificity for the early detection of CCM than conventional echocardiography.59 Although galectin-3 cannot be used as a specific marker of CCM, since it may be secreted by other organs beside the heart, such as the lungs, colon and stomach, it appears to have a greater prognostic value than BNP when assessed separately and, when combined, their prognostic value is even higher.2,60 A study by Yoon et al. of a rat model of CCM reported that galectin-3 inhibitors show some therapeutic potential.61 In their study, inhibition of galectin-3 using N-acetyl-lactosamine decreased cardiac TNF-α and BNP levels and reversed the decreased blood pressure and depressed contractility in the cirrhotic heart.61
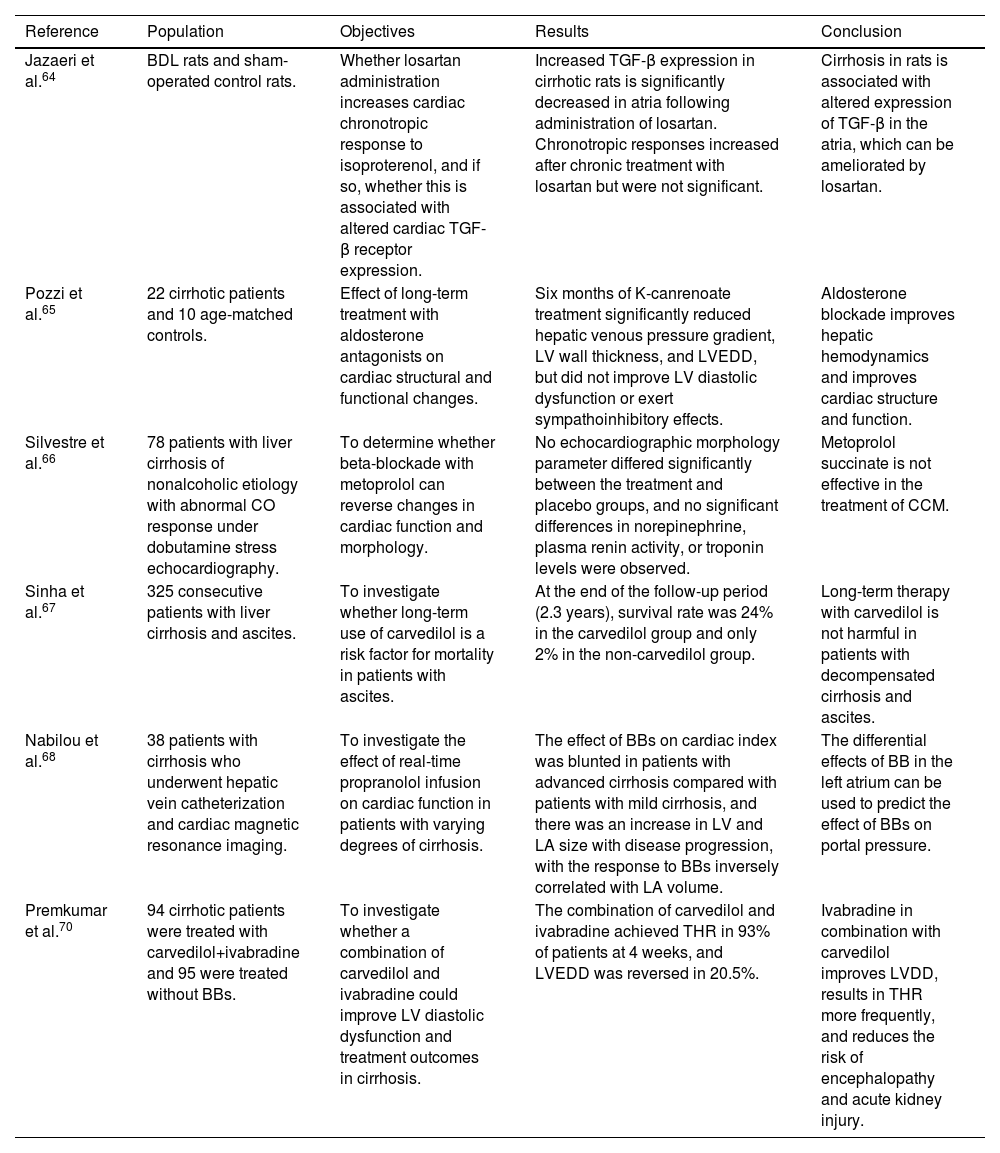
TreatmentThere is still no specific treatment available for CCM. Treatment is mainly supportive and only initiated when HF becomes apparent, following the principles of HF treatment in general, including salt and water restriction, diuretic therapy, and careful preload reduction.28,62 However, many drugs typically used in this context are not successful in cirrhotic patients. Traditional vasodilators may worsen hypotension and hypoperfusion because of decreased peripheral vascular resistance.2,14 Administration of beta-agonists is also unlikely to be helpful in view of the down-regulation of beta-adrenergic receptors in cirrhosis.28 A single-center study that assessed the effect of ouabain on cardiac function in cirrhotic patients suggested that cardiac glycosides might also not be effective in CCM.63 However, the authors speculate that this lack of response could be due to inadequate dosage in the context of a larger extracellular volume in these ascitic patients.63 RAAS inhibitors may also worsen hypotension and are contraindicated in advanced cirrhosis.10,14,28 Studies in animals suggest a potential benefit of losartan in improving adrenergic responsiveness while reducing atrial transforming growth factor-beta expression, but further studies are necessary to understand the possible role of the angiotensin pathway in CCM.64Table 2 presents a summary of studies on pharmacological treatment of cirrhotic cardiomyopathy.
Summary of studies on pharmacological treatment of cirrhotic cardiomyopathy.
| Reference | Population | Objectives | Results | Conclusion |
|---|---|---|---|---|
| Jazaeri et al.64 | BDL rats and sham-operated control rats. | Whether losartan administration increases cardiac chronotropic response to isoproterenol, and if so, whether this is associated with altered cardiac TGF-β receptor expression. | Increased TGF-β expression in cirrhotic rats is significantly decreased in atria following administration of losartan. Chronotropic responses increased after chronic treatment with losartan but were not significant. | Cirrhosis in rats is associated with altered expression of TGF-β in the atria, which can be ameliorated by losartan. |
| Pozzi et al.65 | 22 cirrhotic patients and 10 age-matched controls. | Effect of long-term treatment with aldosterone antagonists on cardiac structural and functional changes. | Six months of K-canrenoate treatment significantly reduced hepatic venous pressure gradient, LV wall thickness, and LVEDD, but did not improve LV diastolic dysfunction or exert sympathoinhibitory effects. | Aldosterone blockade improves hepatic hemodynamics and improves cardiac structure and function. |
| Silvestre et al.66 | 78 patients with liver cirrhosis of nonalcoholic etiology with abnormal CO response under dobutamine stress echocardiography. | To determine whether beta-blockade with metoprolol can reverse changes in cardiac function and morphology. | No echocardiographic morphology parameter differed significantly between the treatment and placebo groups, and no significant differences in norepinephrine, plasma renin activity, or troponin levels were observed. | Metoprolol succinate is not effective in the treatment of CCM. |
| Sinha et al.67 | 325 consecutive patients with liver cirrhosis and ascites. | To investigate whether long-term use of carvedilol is a risk factor for mortality in patients with ascites. | At the end of the follow-up period (2.3 years), survival rate was 24% in the carvedilol group and only 2% in the non-carvedilol group. | Long-term therapy with carvedilol is not harmful in patients with decompensated cirrhosis and ascites. |
| Nabilou et al.68 | 38 patients with cirrhosis who underwent hepatic vein catheterization and cardiac magnetic resonance imaging. | To investigate the effect of real-time propranolol infusion on cardiac function in patients with varying degrees of cirrhosis. | The effect of BBs on cardiac index was blunted in patients with advanced cirrhosis compared with patients with mild cirrhosis, and there was an increase in LV and LA size with disease progression, with the response to BBs inversely correlated with LA volume. | The differential effects of BB in the left atrium can be used to predict the effect of BBs on portal pressure. |
| Premkumar et al.70 | 94 cirrhotic patients were treated with carvedilol+ivabradine and 95 were treated without BBs. | To investigate whether a combination of carvedilol and ivabradine could improve LV diastolic dysfunction and treatment outcomes in cirrhosis. | The combination of carvedilol and ivabradine achieved THR in 93% of patients at 4 weeks, and LVEDD was reversed in 20.5%. | Ivabradine in combination with carvedilol improves LVDD, results in THR more frequently, and reduces the risk of encephalopathy and acute kidney injury. |
BBs: beta-blockers; BDL: bile duct ligated; CCM: cirrhotic cardiomyopathy; CO: cardiac output; LA: left atrial; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; TGF-β: transforming growth factor-beta; THR: targeted heart rate reduction.
A study by Pozzi et al. to assess the effect of treatment with aldosterone antagonists in a group of 22 cirrhotic patients and 10 age-matched controls concluded that a six-month period of K-canrenoate treatment significantly reduced hepatic venous pressure gradient (from 15.3±1.0 to 13.8±0.8 mmHg, p<0.05), LV wall thickness (from 21.8±0.5 to 20.7±0.6 mm, p<0.02) and LV end-diastolic volume (from 99.2±7 to 86.4±6 ml, p<0.01).65 However, this therapeutic intervention neither improved LV DD nor exerted sympathoinhibitory effects.65 The authors propose that an additive effect of beta-blockers (BBs) and aldosterone antagonists plays a role in slowing the progression of CCM.65
Beta-blockadeBBs play an important role in reducing portal hypertension, with a beneficial effect on gastrointestinal variceal bleeding, as well as alleviating the effect of overactivation of the sympathetic system.2 However, their role in the management of CCM remains unclear.
Silvestre and colleagues conducted a prospective randomized trial including 78 patients with cirrhosis of nonalcoholic etiology with abnormal CO response under dobutamine stress echocardiography.66 Patients were divided into two groups and assigned to receive metoprolol or placebo for six months. After this period, no echocardiographic parameter of morphology differed significantly between the two groups, and no significant differences in noradrenaline, plasma renin activity or troponin levels were observed.66 Although this trial did not show that metoprolol succinate was effective in the treatment of CCM, it was limited by a small sample size, and a longer treatment period could have demonstrated some statistically significant benefits.66
Sinha et al.67 performed a retrospective analysis examining cirrhotic patients with and without treatment with carvedilol, a combined alpha- and beta-adrenergic blocker. They found a survival rate of 24% in the carvedilol group and of only 2% in the non-carvedilol group at the end of follow-up (2.3 years).67 This difference was statistically significant (p=0.001) and remained after adjusting for age, etiology of cirrhosis and disease severity. Despite their utility, a 2022 cross-sectional intervention study by Nabilou et al. including 37 cirrhotic patients aiming to investigate the effects of acute beta-blockade on cardiac function found that the impact of BBs on cardiac index was blunted in patients with more advanced cirrhosis compared to those with mild cirrhosis.68 In addition, the same study reported an increase in LV and LA size with disease progression, with response to BBs inversely correlated with LA volume.68 The differential effects of beta-blockade on the left atrium may be used to predict the effect of BBs on portal pressure.68
IvabradineIvabradine has a role in the management of patients with HF with reduced ejection fraction. As shown by the SHIFT trial (conducted in a group of patients admitted to hospital for HF, in sinus rhythm and with HR ≥70 bpm, and on stable background treatment including a BB), when combined with a BB, ivabradine substantially and significantly reduces cardiovascular death and hospital admission for worsening HF.69 In a recent randomized controlled trial in cirrhotic patients by Premkumar et al., there was improvement in cardiac function and decreased encephalopathy and acute kidney injury in the group given a combination of ivabradine and carvedilol compared to the group given carvedilol alone and the group given standard-of-care therapy without any specific intervention.70 Although promising, these results should be interpreted with caution, as the ivabradine dose used in this trial was low (2.5 mg twice daily titrated to a maximum of 7.5 mg twice daily) and none of the known side effects of this drug, such as atrial fibrillation, were observed in the treatment group.70
Liver transplantationLTx remains the gold standard treatment for CCM, as it improves systolic and diastolic dysfunction.1–3,14 However, the factors that determine the reversibility of cardiac abnormalities are still unknown.3,71 QTc prolongation is usually resolved following LTx.44,72 A study by Torregrosa et al. in a group of 40 cirrhotic patients and 15 controls to assess the reversibility of cardiac alterations in liver cirrhosis patients after transplantation showed that LTx leads to regression of ventricular wall thickness, improvement of diastolic function and normalization of systolic response and exercise capacity during stress.9 However, one group of investigators found that in some patients after LTx, the grade of DD on echocardiography worsened.44 The authors speculate that this return of cardiac dysfunction may be associated with the long-term use of immunosuppressive drugs such as calcineurin inhibitors.44
LTx carries a significant risk of perioperative and postoperative complications, including acute HF, myocardial infarction and life-threatening arrhythmias, which presents a significant cardiovascular challenge for these patients.1,2
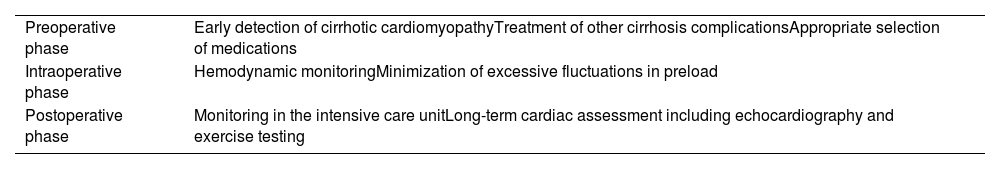
Prior to LTx, patients should be assessed in order to reduce morbidity and mortality associated with cardiac disease, with the help of exercise testing, dobutamine stress echocardiography, stress myocardial perfusion imaging, cardiac computed tomography and coronary angiography.38 Up to 8-30% of patients undergoing LTx will develop post-reperfusion syndrome, associated with a decrease in mean arterial pressure and bradycardia following unclamping of the portal veins and liver reperfusion.10 A 2016 retrospective study including 243 patients who underwent LTx found that the major causes of mortality after LTx were severe infection (39%), cardiac causes (17%), malignancy (14%) and neurological causes (3%).44 Perioperative management in cirrhotic patients should focus on minimizing excessive fluctuations in preload and afterload to minimize the risk of cardiovascular complications, especially precipitation of HF and arrhythmias.38
In 2017, VanWagner et al. developed a point-based risk model (the CAR-OLT score) with the aim of identifying patients at risk for one-year cardiovascular complications after orthotopic LTx.73 Although not validated for the CCM population, it could help to identify patients for whom more intensive cardiovascular testing is needed.73Table 3 presents our recommendations in the peritransplant setting.
Recommendations in the peritransplant setting.
| Preoperative phase | Early detection of cirrhotic cardiomyopathyTreatment of other cirrhosis complicationsAppropriate selection of medications |
| Intraoperative phase | Hemodynamic monitoringMinimization of excessive fluctuations in preload |
| Postoperative phase | Monitoring in the intensive care unitLong-term cardiac assessment including echocardiography and exercise testing |
The impact of CCM on overall survival is still unclear, although given its clinical implications, it should be screened in cirrhotic patients.10 As CCM and cirrhosis progression are correlated, morbidity and mortality due to CCM are difficult to identify as uniquely due to CCM itself.74 A prospective study by Kappor et al. that included 53 cirrhosis patients followed for six months to look for complications and mortality showed a mean survival for patients with and without cardiomyopathy of 5.5 months (95% CI 5.0–5.9) and 5.2 months (95% CI 4.8–5.7), respectively, with the difference between the two groups not being statistically significant.75
CCM also plays an important role in the outcomes of LTx. A single-center retrospective matched cohort study by Izzy et al. including 141 patients undergoing LTx, aiming to assess the impact of CCM on post-transplant cardiovascular outcomes, found that the five-year cardiovascular disease-free survival rate was higher in patients without CCM (85.2% vs. 60.7%), with maximum impact after the first 90 days post-transplant.71 The adverse outcomes in patients with CCM thus require closer post-transplant surveillance and management.71
TIPS insertion can lead to major cardiovascular complications, such as myocardial ischemia, HF, arrhythmias and acute pulmonary edema, which may be the consequence of DD present in cirrhotic patients.10,76 Busk et al. studied a group of 25 patients with cirrhosis referred for TIPS, and found that it led to improvement in inotropic function, whereas chronotropic function remained unchanged.16 Additional studies are needed in order to understand in more detail the impact of this procedure on cardiac function in cirrhotic patients.
In the immediate postoperative period, patients should be carefully monitored for volume status to minimize the risk of cardiovascular complications.38 Shortly after LTx, a complete cardiac workup, including echocardiography and stress testing, should be performed to determine whether cardiac abnormalities have regressed and to better stratify and treat any remaining heart failure.
ConclusionsAlthough recognized for many decades, CCM has been neglected as an academic curiosity and is still poorly understood. Once considered to be a direct effect of cardiac alcohol toxicity, it is now regarded as a major factor for mortality and morbidity in cirrhotic patients, especially following cardiovascular stress events such as TIPS insertion and LTx, while also being implicated in the pathogenesis of HRS following SBP. Impaired beta-receptor responsiveness is heavily involved in the pathogenesis of CCM, as are increased action of NO, carbon monoxide and endocannabinoids, ion channel defects and changes in the constitution of the cardiomyocyte plasma membrane. Since CCM is mainly subclinical, its diagnosis requires a high level of suspicion; cardiac abnormalities include increased CO, low systolic blood pressure, systolic and/or diastolic dysfunction, and electrophysiological abnormalities such as QTc interval prolongation and chronotropic incompetence. To date, there is no specific treatment for CCM and LTx remains the only therapeutic procedure capable of reversing associated cardiac abnormalities. Future clinical trials are needed to establish unified protocols for the management of CCM, as well as to identify beneficial therapies for these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.