Cadmium is a widely distributed toxic heavy metal that has been associated with many diseases including chronic renal dysfunction, osteomalacia, acute heart failure, secondary hypertension, and atherosclerosis. Although several studies have suggested that cadmium may affect multiple systems by inducing lipid per oxidation in cells and disturbing the antioxidant system, the mechanism by which cadmium affects the cardiovascular system remains unclear. Recent studies on heart failure and acute myocardial infarction have shown that cadmium has good predictive ability for mortality in patients with cardiovascular disease. In this study, we briefly review the role of cadmium in cardiovascular disease, which may prompt further studies to investigate the potential association between cadmium and mortality in patients with cardiovascular disease.
O cádmio é um metal tóxico pesado amplamente distribuído que está associado a muitas doenças, inclusive a disfunção renal crónica, a osteomalacia, a insuficiência cardíaca aguda, a hipertensão secundária e a aterosclerose. Embora vários estudos tenham sugerido que o cádmio pode afetar sistemas múltiplos através da indução da perioxidação dos lípidos nas células e da distribuição do sistema antioxidante, o mecanismo através do qual o cádmio afeta o sistema cardiovascular permanece pouco claro. Estudos recentes sobre insuficiência cardíaca e sobre enfarte agudo do miocárdio demonstraram a capacidade preditiva deste metal na mortalidade em doentes com doença cardiovascular. Neste estudo, apresentamos uma breve revisão do papel do cádmio na doença cardiovascular que poderá num futuro próximo motivar novos estudos para investigação da associação potencial entre o cádmio e a mortalidade em doentes com patologia cardiovascular.
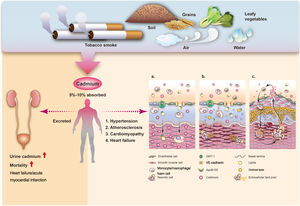
Cadmium is a toxic heavy metal that has been associated with many diseases. Cadmium production increased during the 20th century as a result of the production of nickel-cadmium batteries, metal coatings and plastic stabilizers.1 Exposure to cadmium results from the intake of contaminated food, including leafy vegetables and grains, and drinking water, or by inhaling polluted air.2 Intestinal absorption of cadmium is greater with iron, calcium, or zinc deficiency.3,4 Tobacco smoke is considered to be the most significant source of cadmium exposure, and smokers have approximately twice the cadmium body burden of nonsmokers.5,6 Cadmium is efficiently retained in organs including the kidneys, liver, bone, lung, central nervous system and cardiovascular system. Cadmium accumulates throughout life, with a clearance half-life of 25 years. Accordingly, it can disrupt several biological systems, usually at a lower dose than other toxic metals, such that its deleterious effects can occur at levels as low as 0.5 μg/g creatinine.7
Cadmium absorption and distributionCadmium is absorbed primarily through inhalation and ingestion, and 10-50% of inhaled cadmium dust is absorbed, depending on particle size. About 5-10% of ingested cadmium is absorbed, also depending on particle size.4 After absorption, cadmium is transported throughout the body in blood bound mainly to metallothionein, a sulfhydryl group-containing protein.8 Metallothionein is a heavy metal-binding protein that can protect against heavy metal toxicity and other oxidative stress. Cadmium then accumulates in the human body, mainly in the kidneys and liver, possibly due to the ability of the kidneys and liver to synthesize metallothionein. There is no efficient excretory mechanism for cadmium in the body.
Clinical toxicity of cadmium in systems other than the cardiovascular systemThe main target organ of toxic impact in humans is the kidney, and cadmium will accumulate in the S1 segment of proximal tubules causing Fanconi syndrome, which is a deficiency in reabsorption of proteins, amino acids, glucose, and phosphates.9 Low-level cadmium exposure increases the risk of chronic kidney disease, which is a well-known risk factor for cardiovascular disease (CVD), and thus this may also play a role in cadmium-associated CVD.10 Cadmium will also impair vitamin D metabolism in the kidneys, causing bone deficiency.11 Furthermore, cadmium may directly inhibit gut absorption of calcium and cause derangement of collagen metabolism, which can result in osteomalacia and osteoporosis.4 This is the process that caused itai-itai disease during the mass cadmium poisoning in Toyama Prefecture, Japan, and leads to severe pain from osteomalacia with osteoporosis, renal tubular dysfunction, anemia, and calcium malabsorption.12
Cadmium - epidemiology and pathophysiology in cardiovascular diseaseChronic cadmium exposure has been associated with hypertension, promotion of atherosclerosis and impaired cardiac function.13 The exact effects of cadmium on the cardiovascular system are controversial. However, studies have shown that it may have adverse effects on the cardiovascular system at extremely low doses. An in vitro study showed that a dose of cadmium well below toxic concentrations may initiate pathological changes in vessel walls.14
Epidemiological evidence of cadmium's cardiovascular effectsThe cardiovascular effect of cadmium has been observed in in vitro studies and experimental animal models.15 People living in areas with higher potential for cadmium exposure have been reported to have an increased risk of cardiovascular mortality.16,17 A study examining data from the US National Health and Nutrition Examination Surveys (NHANES) reported an association between serum cadmium levels and elevated blood pressure. Moreover, the association was stronger among non-smokers, intermediate among former smokers, and small or null among current smokers.18 Ruiz-Hernandez et al. reported that a decline in environmental cadmium exposure was associated with a reduction in cardiovascular mortality in the US.19 In addition, Tellez-Plaza et al. reported that the pooled relative risks (95% confidence interval [CI]) for CVD, coronary heart disease, stroke, and peripheral arterial disease due to cadmium exposure were 1.36 (95% CI: 1.11-1.66), 1.30 (95% CI: 1.12-1.52), 1.18 (95% CI: 0.86-1.59), and 1.49 (95% CI: 1.15-1.92), respectively. Moreover, the pooled relative risks for cardiovascular disease in men, women and non-smokers were 1.29 (95% CI: 1.12-1.48), 1.20 (95% CI: 0.92-1.56) and 1.27 (95% CI: 0.97-1.67), respectively.20 Although women have a higher cadmium burden than men, the risk of cardiovascular disease-related mortality is lower in women.16 This may be due to differences in estrogen between men and women.21
Different cadmium biomarkers may provide different information about the timing and source of exposure. The use of biomarkers has been inconsistent across epidemiological studies. However, generally speaking, urinary cadmium levels reflect the body burden due to long-term exposure, and serum cadmium level is considered to represent recent exposure.22
Basic science of cadmium and heart diseaseThe form in which cadmium is taken up by cells is still unclear, and further studies are needed to elucidate the expected amount in the circulation and the route of cadmium uptake in cells. However, several cellular transporters and ion channels have been reported to transport cadmium across the cell membrane, including calcium channels.23 Cadmium may also be taken up by immune system cells and infiltrate into vessel walls through cadmium-laden monocytes.24 Due to the critical roles of monocytes and macrophages in transdifferentiation into foam cells and necrotic foam cell death in endothelial dysfunction, their excessive production due to cadmium may play an important role in the initiation and promotion of atherosclerosis. Cadmium can disrupt endothelial integrity and cause cadmium-mediated endothelial cell death. The formation of gaps between endothelial cells may allow cadmium to diffuse from the bloodstream into the media layer.25 Cadmium is mainly retained in smooth muscle cells after transport across endothelial cells. Its effects on smooth muscle cells include interactions with ion homeostasis and calcium ion flux, cytotoxic effects, and stimulation of smooth muscle cell proliferation at low cadmium concentrations, thereby allowing for subsequent lipid accumulation in vessel walls and modification of lipid profiles toward a more atherogenic state.26,27 Cadmium-induced endothelial cell death is thought to be the fundamental process by which cadmium promotes atherosclerosis, damaging the integrity of the vascular endothelium, and thereby also contributing to vascular inflammation.28 Cytokines and overactivated inflammation reactions play an important role in atherosclerosis.29 Cadmium accumulation increases several important pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor alpha, which may affect inflammation in atherosclerosis.30,31 Moreover, a study of ApoE-/- mice showed that chronic cadmium exposure increased the level of total cholesterol and decreased acetylcholine relaxation, thereby reducing nitric oxide bioavailability.32 These studies suggest possible mechanisms by which cadmium increases the risk of atherosclerosis and causes cardiovascular damage. The mechanism of cadmium-induced atherosclerosis is illustrated in Figure 1.
Illustration of the relationship between cadmium and cardiovascular disease, from absorption (top) to excretion (lower left), and the potential mechanisms of increasing atherosclerosis and major adverse events (lower right). Cadmium may be transported into endothelial cells of vessel walls, including passive and/or active transport through membrane channels, paracellular diffusion (1a), causing endothelial cell death and inflammation. Other processes lead to accelerated atherosclerosis through the monocyte-macrophage lineage, and result in oxidative stress and lipid oxidation in the vessel wall. Foam cells secreting cytokines promote inflammation (1b) and atherosclerotic plaque formation (1c).
Although the mechanisms underlying the association between cadmium and heart failure (HF) remain unknown, several experimental and epidemiological studies have provided some insight. Rat muscle cells have been shown to be deformed by cadmium due to an increase in free radicals and lipid peroxidation.33 Another study showed that cadmium may inhibit heart pyruvate-malate-supported mitochondrial respiration, suggesting that disturbances in myocardial metabolism and function may occur after cadmium exposure.34
Cadmium and coronary heart diseaseA cross-sectional analysis of the 1988-1994 NHANES surveys showed that urinary cadmium ≥0.88 μg/g creatinine had an odds ratio (OR) of 1.86 (95% CI: 1.26-2.75) compared to urinary cadmium <0.43 μg/g creatinine for acute myocardial infarction (AMI) and an OR of 1.80 (95% CI: 1.06-3.04) compared to urinary cadmium <0.43 μg/g creatinine.35 A meta-analysis of data from prospective studies on the association between cadmium and coronary heart disease (CHD) showed that higher blood cadmium levels are associated with increased CHD mortality, with a hazard ratio of 1.60 (95% CI 1.21-2.10).36
Cadmium and heart failureAmong the etiologies of cardiomyopathy, dilated cardiomyopathy (DCM) is the most common.37 Although cadmium is mainly concentrated in the liver and kidneys, exposure to low doses can result in a significantly increased level in the heart.38 Several studies have suggested that cadmium plays a role in the pathogenesis of DCM, and cadmium blood and urine levels have been shown to be significantly elevated in patients with DCM compared to controls. Importantly, control patients with DCM have been shown to exhibit a two-fold increase in blood cadmium levels compared with smokers, ex-smokers, and non-smokers.39
An epidemiological study of 12 049 US participants demonstrated that urinary cadmium levels were significantly correlated with the self-reported prevalence of HF.40 A prospective cohort study of 3348 adults in the US also revealed a significant association between urinary cadmium and increased incidence of HF, with a corresponding hazard ratio for HF of 1.39 (95% CI: 1.01-1.94).41 Meanwhile, a study of 4378 participants in Sweden with a 17-year follow-up period demonstrated that participants in the fourth quartile of serum cadmium levels had a significantly higher incidence of HF than those in the first quartile.42 Taken together, these studies suggest that cadmium could be directly correlated with HF.
Cadmium and strokeCadmium exposure is associated with prevalence and inflammation of carotid plaques, and an increased risk of ischemic stroke.43–45 A study of 599 64-year-old women demonstrated that blood and urine cadmium levels were associated with the development of atherosclerotic plaques in carotid arteries.43 In addition, an epidemiological study of 4639 middle-aged women and men in Sweden confirmed an association between cadmium exposure and atherosclerosis.44 Another study in Sweden showed a significant interaction between cadmium and ischemic stroke.46 Similar findings have been reported in the US, where a study of 680 ischemic stroke cases and 2540 participants suggested that urinary cadmium was associated with an increased incidence of ischemic stroke.47
Cadmium and peripheral arterial diseaseThe vascular wall is a target of cadmium deposition.48 As coronary artery disease (CAD) shares similar risk factors with peripheral arterial disease (PAD), cadmium accumulation may also affect PAD. A NHANES study reported that PAD may be associated with serum and urinary cadmium, suggesting a positive association between cadmium exposure and CVD.49 Another prospective cohort study reported that urinary cadmium was independently associated with the incidence of PAD.50 Tellez-Plaza et al. also analyzed data from NHANES, and showed that higher blood and urine cadmium levels were associated with an increased prevalence of PAD.51 Moreover, cadmium not only increased the prevalence of PAD but was also associated with its severity. A cross-sectional study of patients with CAD and PAD showed that cadmium accumulation may be a quantitative risk factor for the development of symptomatic PAD of the lower extremities: from low cadmium burden in CAD only to high cadmium burden in CAD with critical limb ischemia.52
Cadmium - a potential powerful biomarker to predict prognosis in cardiovascular diseaseThe urinary excretion of cadmium is known to be closely correlated with cadmium accumulation in the human body.53 Studies have shown that urinary excretion of cadmium not only reflects the body's cadmium burden, but can also be used to predict mortality in patients with HF and AMI, as discussed below.
Urinary cadmium levels predict mortality in patients with acute heart failureAcute HF is a serious condition associated with high morbidity and mortality in critically ill patients.54 Several scoring systems have been used to evaluate illness severity and predict mortality for HF patients, including the Sequential Organ Failure Assessment (SOFA), Acute Physiology and Chronic Health Evaluation II (APACHE II), and the Multiple Organ Dysfunction Score.55–57 Although these scoring systems are widely used in clinical practice, they are complicated and time-consuming, and there may be significant interobserver variability among the physicians who calculate the scores.40 Therefore, a single and reliable predictor for mortality is needed for critically ill patients, as these patients often need prompt and aggressive treatment. We previously showed that urinary cadmium levels on day 1 (D1UCd) had a high discriminatory power and good predictive ability for mortality in patients with acute HF. Furthermore, D1UCd was better than established predictive scoring systems, because it is a single objective variable which has no interobserver variability among physicians.58 Therefore, D1UCd may be a single predictor of mortality in patients with acute HF.
Urinary cadmium levels can predict 30-day mortality and illness severity in patients with acute myocardial infarctionA previous study reported the predictive ability of cadmium for 30-day mortality and illness severity in patients with AMI.59 The 30-day mortality rate after AMI has been shown to be an indicator of the appropriateness and effectiveness of diagnostic and therapeutic procedures for patients with AMI after hospital admission.60 The work by Lin et al. showed that D1UCd was significantly and independently associated with mortality in AMI patients, and that each 1-μg increase in D1UCd was associated with a 1.5-fold increased risk of 30-day mortality in these patients.59 Previous clinical studies have also shown elevated serum cadmium levels in patients in the acute phase of AMI and without a history of cadmium exposure.61 Although the mechanism underlying the association between cadmium and mortality in AMI patients is still unknown, several studies indicate that cadmium is directly correlated with atherosclerosis, which is the major risk factor for AMI. Furthermore, a previous study showed that nitric oxide can displace cadmium from metallothionein.62 When the body faces oxidative stress such as AMI, the inducible nitric oxide synthase system in the vascular endothelium is activated and increases the production of nitric oxide.63 This increase in nitric oxide may cause the release of cadmium from a stable binding form in the vascular endothelium and liver, thereby causing damage and being excreted in urine in patients with AMI. However, in light of recent research, we think that direct cell toxicity from cadmium is the most likely hypothesis.
There are several limitations to the use of cadmium as a predictor of mortality in patients with severe CVD. Although D1UCd has been significantly associated with mortality in patients with acute HF and AMI, the causal relationship is uncertain.64 In addition, the two previous studies which reported associations between D1UCd and HF and AMI mortality were conducted at a single institution, and the results may not be applicable to other hospitals. Therefore, a multicenter investigation with more patients is needed. Due to concomitant exposure to other heavy metals such as lead and mercury, further studies are needed to investigate interactions between these metals and their role in mortality in patients with HF and AMI. Furthermore, studies are warranted to investigate whether daily urine cadmium excretion is useful for assessing treatment in patients with AMI and HF, and whether chelation of cadmium will reduce mortality.
Cadmium - insights from chelation trialsOver recent decades, although its indications and prevalence remain unknown, chelation practitioners have increasingly used ethylenediaminetetraacetic acid (EDTA) for CAD and PAD.65 The Trial to Assess Chelation Therapy (TACT) was a double-blind, placebo-controlled randomized trial enrolling 1708 patients aged 50 years or older who had experienced AMI at least six weeks previously. The results showed that compared with placebo, stable patients with a history of AMI treated with EDTA had a modestly reduced risk of adverse cardiovascular outcomes.66
ConclusionsThe association between cadmium and cardiovascular disease has been proved, however the pathophysiological mechanism has yet to be verified. Based on the good correlation between urinary excretion and accumulation of cadmium in the human body, urinary cadmium levels may predict mortality in patients with HF or AMI. D1UCd has been shown to have good predictive ability and discriminatory power for mortality in these patients, and to outperform established predictive scoring systems with minimal interobserver differences. Further investigations are needed to clarify these observations.
Conflicts of interestThe authors have no conflicts of interest to declare.