The authors report the first catheter ablation of Brugada syndrome in the literature using the Rhythmia™ mapping system. Learning points include: (1) low voltage areas can be documented while mapping in some individuals, suggesting that Brugada syndrome may not be a pure ion channel disorder; (2) typical long fractionated potentials can also be identified in the endocardium, supporting the need to map the endocardium in all Brugada patients requiring ablation; (3) disappearance of the typical coved pattern following ablation does not necessarily predict cure, as the patient we present experienced ventricular fibrillation recurrence a few months later.
Os autores descrevem a primeira ablação de síndrome de Brugada usando o sistema de mapeamento Rhythmia™. Pontos de aprendizagem incluem: 1) é possível observar fibrose ao mapear alguns destes indivíduos, sugerindo que a síndrome de Brugada poderá não ser uma canalopatia pura; 2) os potenciais fracionados de longa duração também podem ser identificados no endocárdio, reforçando a necessidade de mapeamento também do endocárdio em todos os doentes com Brugada com indicação para ablação; 3) desaparecimento do padrão típico coved não prevê necessariamente a cura, uma vez que no caso apresentado uma recidiva de fibrilhação ventricular foi observada alguns meses mais tarde.
A 39-year-old man with Brugada syndrome (BS) and recurrent arrhythmia storms (nearly once a month) due to ventricular fibrillation (VF) resistant to maximum tolerated doses of quinidine was referred for catheter ablation in our center. His heart was structurally normal, with normal left and right ventricular systolic function and no chamber dilatation or ventricular wall motion abnormalities on recent echocardiographic assessment or MRI performed some years before. He had received an implantable cardioverter-defibrillator following an episode of out-of-hospital ventricular fibrillation.
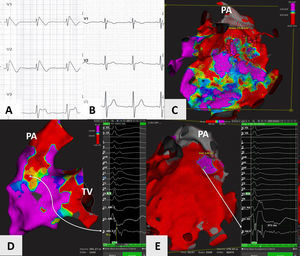
The patient presented with spontaneous type 1 pattern at the start of the procedure (Figure 1A), and both endocardial and epicardial access was obtained. The Rhythmia™ mapping system (Boston Scientific©, MA, USA) was used, and after mapping with the Intellamap Orion™ catheter (Boston Scientific©, MA, USA), fractionated potentials were targeted using the Intellanav™ open-irrigated catheter (Biosense Webster©, CA, USA) (Figure 1C). Some fractionated potentials were found in the right ventricular outflow tract (RVOT) endocardium (Figure 1D), but longer potentials, measuring >200 to 300ms, were observed, mostly in the epicardial layer (Figure 1E) (potential duration was measured from start to end of the local electrogram using the method described by Pappone et al.1). Interestingly, areas of scar were also detected in the RVOT endocardium and epicardium (Figure 1C and D). Following ablation in the area (40W, 30 s per lesion until eradication of the long-duration potentials), the coved type 1 pattern disappeared. After nine months of follow-up, and no recurrence of the coved type 1 pattern (Figure 1B), the patient had a single episode of VF in the second month, and is now free from anti-arrhythmic drugs.
(A and B) Precordial leads (V1 to V3) showing presence of spontaneous type 1 Brugada pattern at the start of the procedure, and its disappearance nine months later; (C) epicardial voltage map of the right ventricle using the Rhythmia™ mapping system, anteroposterior view with 0.5 and 1.5mV as voltage cut-off points, showing large areas of fibrosis in the right ventricular outflow tract (RVOT) and anterior wall of the right ventricle; (D) endocardial voltage map of the right ventricle, posteroanterior view, showing an area of fibrosis in the posteroseptal aspect of the RVOT. A long (>120ms), complex, small-amplitude and fractionated potential is highlighted; (E) potential duration map of the epicardial aspect of the right ventricle, anteroposterior view, showing area of potentials lasting more than 291ms (purple), corresponding to the ablated zone. Areas of potentials lasting less than 200ms are in red. Highlighted is a very long and complex potential measuring approximately 371ms. PA: pulmonary artery; TV; tricuspid valve.
This first case of BS ablation using the Rhythmia™ mapping system illustrates three important points. Firstly, fibrosis can also be present in some individuals with BS who do not meet criteria for arrhythmogenic right ventricular dysplasia. This had been suggested by Coronel et al.2 Whether fibrosis results from a more aggressive form of channelopathy in which channel dysfunction leads to tissue changes, additional mutations or polymorphisms in different genes, or modulators leading to fibrosis, or from a coincidental occurrence of localized myocarditis, remains to be determined. In this case no mutation or polymorphism could be identified, and therefore we cannot provide data to support the above hypothesis. However, a recent publication suggests that BS patients with SCN5A mutations may exhibit more conduction abnormalities on the electrocardiogram and be at higher risk for cardiac events.3 Nademanee et al. have shown that increased collagen, fibrosis and reduced gap junction signaling protein Cx43 expression in the RVOT are present in BS patients.4 These data support the plausibility of BS reflecting a generalized disease of myocardial architecture, and the baseline properties of the RVOT which predispose it to fibrosis are likely to contribute to the condition and its arrhythmic risk. The presence of coexistent fibrosis may explain why up to 5% of BS patients may also develop monomorphic ventricular tachycardias.5 Secondly, our findings of fractionated potentials in the endocardium (Figure 1D) suggest there may still be a rationale for seeking ventricular ectopics originating from the area or for ablating long-duration endocardial potentials.6 In our case, as these potentials were only identified on retrospective analysis of the map and were therefore not ablated, we cannot help but wonder if the preservation of this zone is the reason why the patient relapsed. A recent investigation using simultaneous non-invasive mapping of the endocardium and epicardium suggests that both appear to be involved in a substantial number of patients, and their interplay appears to play a role in the observed phenotype.7 Also, in our case, prolonged endocardial electrograms were located in the low posteroseptal region of the RVOT, which is not the traditional area of epicardial involvement in BS. Thirdly, disappearance of the coved pattern does not necessarily predict cure. In this case we hypothesize that several factors may have contributed to VF recurrence, including the existence of an underlying fibrotic substrate (which may have been aggravated with ablation) and a low transmyocardial repolarization gradient which was insufficient to result in a coved type 1 pattern, but in the presence of an appropriately timed ventricular ectopic was still sufficient to degenerate into VF. Finally, much remains to be learned about BS, and the growing number of catheter ablations for this disease will certainly help expand our knowledge.
The high-density mapping capabilities of the Rhythmia™ mapping system are advantageous in this setting as very precise mapping, with no limit to the number of collected points, and identification of the culprit potentials is likely to have an impact on procedural success and prognosis in these patients. Furthermore, if the future of mapping in this condition is to follow the approach used by Pappone et al., 1 in which mapping is repeated following ablation and repeated ajmaline infusion, the ultra-rapid mapping potential of the Rhythmia™ system will make it a likely candidate in this setting.
With regard to the use of this system in the epicardium, we created a shell comprising two layers, one corresponding to the visceral epicardium adjacent to the myocardium, and the other to the parietal pericardium. The analysis presented in these images is only of the visceral epicardium, as we excluded the outer layer of the shell.
The definition that can be obtained with high-density mapping is very different from that of point-by-point mapping using other mapping systems. For example, our epicardial map was composed of 50 472 electrograms, which is >100 times more detailed than was described in a previous case report, in which only 304 points were collected.8 Unfortunately, unlike ours, this case,8 which displayed a right bundle branch block pattern at baseline, and the presence of Brugada pattern in the right precordial and also in the inferior limb leads, did not present follow-up data beyond six weeks.
In our case, we did not perform post-ablation ajmaline testing or programmed ventricular stimulation. The controversy regarding programmed ventricular stimulation in the setting of risk assessment has lasted for decades. Even though it has been used by some authors,1,8 its role as a predictor of success of catheter ablation is still open to debate.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.