Brugada syndrome (BrS) is a channelopathy associated with ventricular arrhythmias and sudden cardiac death. In patients at high risk of sudden death, an implantable cardioverter-defibrillator is indicated. Subcutaneous implantable cardioverter-defibrillators (S-ICDs) are an alternative to transvenous systems, with reduced risk of infection and complications associated with system extraction or explantation.
ObjectiveTo test electrocardiographic eligibility for S-ICD placement after exercise stress testing (EST) in patients with BrS.
MethodsThe sample included 35 consecutive patients with BrS. Electrocardiographic eligibility was assessed using the Boston Scientific model 2889 EMBLEM™ S-ICD automated screening tool, in four phases: decubitus and orthostatism, and before and after EST. Those who had at least one acceptable vector in the four measurements were considered eligible.
ResultsIn this study, 71.4% of patients were male and mean age was 53.86±12 years. In screening prior to EST, 14.3% of patients (n=5) were not eligible for an S-ICD. There was a statistically significant association between ineligibility and presence of complete right bundle branch block and history of syncope. After EST, 16.7% of initially eligible patients no longer had eligible vectors (n=5).
ConclusionIn this study, 16.7% of patients previously eligible for an S-ICD were no longer eligible after EST. This result demonstrates the importance of screening after EST in all patients with BrS and with indication for an S-ICD, and may influence decisions concerning which ICD to implant or whether to institute pharmacological measures that avoid inappropriate therapies.
A síndrome de Brugada (SBr) é uma canalopatia associada ao desenvolvimento de arritmias ventriculares e morte súbita cardíaca. Pacientes com alto risco devem ser orientados para a colocação um cardioversor-desfibrilhador. O cardioversor-desfibrilhador implantável subcutâneo (CDI-S) é uma alternativa ao sistema transvenoso, com menor risco de infeção e complicações associadas à extração/explante do sistema.
ObjetivosTestar a elegibilidade eletrocardiográfica para colocação de CDI-S após prova de esforço (PE) em pacientes com SBr.
MétodosA amostra incluiu 35 pacientes, consecutivos, com SBr. A elegibilidade eletrocardiográfica foi avaliada pela ferramenta de rastreio automática, Boston-Emblem S-ICD (Modelo 2889)®, em quatro fases: decúbito e ortostatismo, antes e depois da PE – foram considerados elegíveis indivíduos que tinham pelo menos um vetor aceitável nas quatro fases.
ResultadosNeste estudo, 71,4% dos pacientes eram do sexo masculino, com idade média de 53,86±12 anos. No rastreio anterior à PE, 14,3% dos pacientes (n=5) não eram elegíveis para o implante de CDI-S. Demonstrou-se uma associação significativa entre não elegibilidade e a presença de bloqueio de ramo direito e história de síncope. Após a PE, 16,7% dos pacientes inicialmente elegíveis deixaram de ter vetores aceitáveis para a implantação de CDI-S (n=5).
ConclusãoNeste estudo, 16,7% dos pacientes previamente elegíveis para CDI-S deixaram de o ser após a PE. Estes resultados demonstram a relevância do rastreio após PE em todos os pacientes com SBr e com indicação para CDI-S, influenciando a decisão do sistema a implantar ou medidas farmacológicas que evitem casos de terapias inadequadas.
Brugada syndrome (BrS) is a genetic disease associated with ventricular arrhythmias (VA) and is responsible for 4-12% of cases of sudden cardiac death (SCD).1 It is characterized by an electrocardiographic (ECG) pattern of ST-segment elevation with type 1 morphology in the right precordial leads V1-V3.2
It is accepted that appropriate use of an implantable cardioverter-defibrillator (ICD) in high-risk patients with aborted SCD and hemodynamically compromising arrhythmias is life-saving. However, there remains a lack of consensus on the management of patients with BrS and no history of VA or aborted SCD, especially in the context of a resting type 1 coved ECG pattern.3
Current guidelines recommend ICD implantation in patients with BrS with spontaneous type 1 ECG pattern and probable arrhythmia-related syncope.4
Nevertheless, whether other clinical factors are better predictors or facilitate more refined risk stratification before any arrhythmic event is still up for debate. This is especially important as the first clinical event may be cardiac arrest.3
Transvenous ICD (TV-ICD) systems have been associated with high complication rates in BrS patients, including higher defibrillation thresholds, inappropriate shock rates, and lead failure rates.5 A subcutaneous ICD (S-ICD) can be an effective alternative to a conventional TV-ICD system in patients with BrS with a lower risk of complications.6 S-ICD systems do not require placement of leads directly in the heart, so these devices could avoid the complications related to the use of TV-ICD leads. Moreover, since the incidence of lead injury increases over time after TV-ICD implantation, use of S-ICDs is expected to avoid problems with cardiac leads, especially in younger patients without organic heart disease, such as patients with BrS, who do not usually need ventricular pacing.2
However, the morphology-based sensing algorithm of the S-ICD is vulnerable to cardiac oversensing.5 To avoid this problem, a system was developed to identify patients who are likely to be unsuitable for an S-ICD, using supine and standing surface ECG screening templates. Nevertheless, ST-T morphology shows fluctuations, particularly in high-risk patients with BrS, and exercise is one of the most important factors for such fluctuations. Exercise-induced ST-T changes thus need to be considered when making decisions regarding S-ICD implantation.2
Our aim is to test electrocardiographic eligibility for S-ICD placement after exercise stress testing (EST) in patients with BrS.
MethodsA cohort of 35 patients diagnosed with BrS, selected from arrhythmology consultations between 2007 and 2019, were analyzed.
BrS was diagnosed in the presence of spontaneous type 1 ECG pattern or of type 2 or type 3 when provocative testing with intravenous administration of a class I antiarrhythmic drug induced a type 1 ECG morphology.7
The only exclusion criterion was prior implantation of an S-ICD. The hospital's ethics committee approved all study protocols.
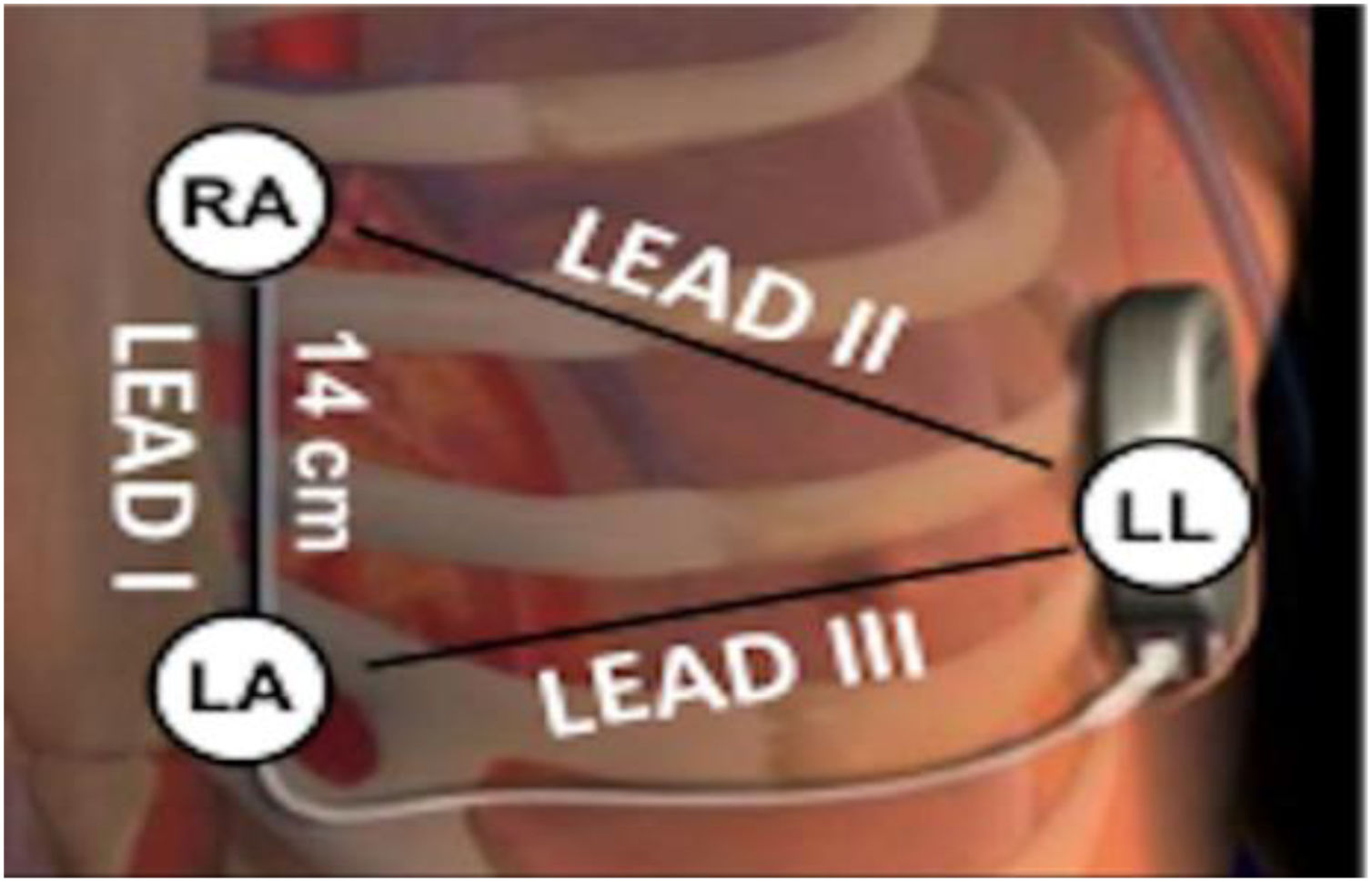
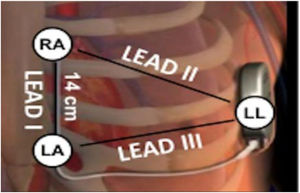
Electrocardiographic eligibility was assessed using the Boston Scientific model 2889 EMBLEM™ S-ICD automated screening tool (AST). ECG leads were placed in the standard manufacturer's configuration for the Boston Scientific S-ICD system (Figure 1). The electrodes were placed 1 cm lateral to the xiphoid process, 14 cm cranial to the xiphoid process on the chest wall, and in the fifth or sixth intercostal space on the left midaxillary line. A ground electrode was placed on the clavicle or in a soft tissue location on the right leg. This electrode configuration was designed to mimic the sensing vectors available on the S-ICD.
Diagram of subcutaneous implantable cardioverter-defibrillator lead vectors and placement of surface electrodes (white circles) during screening. The alternate lead vector extends from 1 cm left lateral of the xiphoid process (LA) to the fifth or sixth intercostal space along the left mid-axillary line (LL). The secondary lead vector extends from 14 cm cranially to the LA (RA) to the LL. The primary lead vector extends from RA to LA (images courtesy of Boston Scientific Corporation).
AST was used in decubitus and orthostatism in all patients before EST to test their eligibility.
Patients were considered eligible in this first phase if they had at least one acceptable vector in the two measurements, and then eligibility was tested again after symptom-limited treadmill exercise testing using the Bruce protocol, also in orthostatism and dorsal decubitus, at the beginning of the recovery phase.
In addition, the participants’ clinical characteristics were assessed, including gender, age, body mass index, clinical manifestations, family history of sudden death, cardiovascular risk factors, usual medication, genetic analysis, ECG parameters and echocardiogram. Body temperature was measured at the beginning of the recovery phase.
The statistical analysis was performed using IBM SPSS 25.0 (IBM SPSS®, Armonk, NY, USA). Categorical variables were expressed as percentages and analyzed using Pearson's chi-square and Fisher's test as appropriate. Continuous variables were expressed as means with standard deviation or medians with interquartile range and compared between groups using the Student's t test or the Mann-Whitney test. Values of p<0.05 were considered significant.
ResultsThirty-five randomly selected patients with a diagnosis of BrS underwent baseline ECG screening for an S-ICD (before EST). Of these, 71.4% (n=25) were male and mean age was 53.86±12 years.
Brugada type 1 ECG pattern was documented spontaneously in 20 patients (57.1%) and was induced by ajmaline challenge in the other 15 (42.9%).
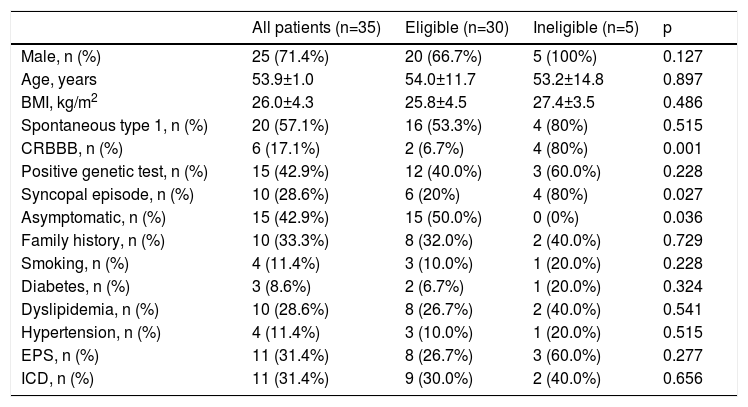
In screening with the Boston Scientific AST prior to EST, 14.3% of patients (n=5) were not eligible for an S-ICD. There was a statistically significant association between ineligibility and presence of complete right bundle branch block (CRBBB) (80 vs. 6.7%, p<0.001%) and presence of symptoms potentially associated with dysrhythmias (50 vs. 100%, p=0.036), particularly history of syncope (80 vs. 20%, p=0.027). No significant differences in other baseline clinical characteristics were seen between patients eligible and ineligible for S-ICD screening (Table 1).
Baseline characteristics of the study population.
| All patients (n=35) | Eligible (n=30) | Ineligible (n=5) | p | |
|---|---|---|---|---|
| Male, n (%) | 25 (71.4%) | 20 (66.7%) | 5 (100%) | 0.127 |
| Age, years | 53.9±1.0 | 54.0±11.7 | 53.2±14.8 | 0.897 |
| BMI, kg/m2 | 26.0±4.3 | 25.8±4.5 | 27.4±3.5 | 0.486 |
| Spontaneous type 1, n (%) | 20 (57.1%) | 16 (53.3%) | 4 (80%) | 0.515 |
| CRBBB, n (%) | 6 (17.1%) | 2 (6.7%) | 4 (80%) | 0.001 |
| Positive genetic test, n (%) | 15 (42.9%) | 12 (40.0%) | 3 (60.0%) | 0.228 |
| Syncopal episode, n (%) | 10 (28.6%) | 6 (20%) | 4 (80%) | 0.027 |
| Asymptomatic, n (%) | 15 (42.9%) | 15 (50.0%) | 0 (0%) | 0.036 |
| Family history, n (%) | 10 (33.3%) | 8 (32.0%) | 2 (40.0%) | 0.729 |
| Smoking, n (%) | 4 (11.4%) | 3 (10.0%) | 1 (20.0%) | 0.228 |
| Diabetes, n (%) | 3 (8.6%) | 2 (6.7%) | 1 (20.0%) | 0.324 |
| Dyslipidemia, n (%) | 10 (28.6%) | 8 (26.7%) | 2 (40.0%) | 0.541 |
| Hypertension, n (%) | 4 (11.4%) | 3 (10.0%) | 1 (20.0%) | 0.515 |
| EPS, n (%) | 11 (31.4%) | 8 (26.7%) | 3 (60.0%) | 0.277 |
| ICD, n (%) | 11 (31.4%) | 9 (30.0%) | 2 (40.0%) | 0.656 |
BMI: body mass index; CRBBB: complete right bundle branch block; EPS: electrophysiological study; ICD: implantable cardioverter-defibrillator; Spontaneous type 1: history of spontaneous type 1 Brugada morphology on 12-lead electrocardiogram.
After EST, 16.7% (n=5) of initially eligible patients no longer had eligible vectors. This second screening was performed with the AST, at the beginning of the recovery phase in orthostatism and dorsal decubitus.
No statistically significant differences were found between eligible and ineligible individuals after EST in any of the parameters analyzed.
Nevertheless, 48% (n=12) of patients had a reduction of eligible vectors after EST, even if they still had at least one eligible vector. There was a correlation between this reduction and the patient's age (47.25 vs. 60.23 years; p=0.004) and maximum heart rate (HR) (157.67 vs. 138.67 bpm; p=0.005).
Considering patients who were ineligible prior to EST and those who became ineligible after EST, there were a total of 28.6% (n=10) patients who had no suitable vectors for S-ICD placement.
DiscussionRisk stratification in BrS is problematic due to the lack of randomized controlled trials. According to the latest European guidelines, ICD implantation is recommended in BrS patients who are survivors of cardiac arrest and/or have documented spontaneous sustained ventricular tachycardia (class I); can be useful in patients with a spontaneous diagnostic type 1 electrocardiogram who have a history of syncope judged to be likely caused by VA (class IIa); and may be considered in patients with a diagnosis of BrS who develop ventricular fibrillation (VF) during programmed electrical stimulation (inducible patients) (class IIb).7
Despite the value of ICDs, TV-ICD therapy has several disadvantages for BrS patients. In a recent meta-analysis, Olde Nordkamp et al. showed that TV-ICDs carry a significant risk of inappropriate shocks and in-hospital and post-discharge complications in relatively young patients with inherited arrhythmia syndromes, especially BrS, with an annual inappropriate shock rate of 3.9%, predominantly due to supraventricular tachycardia and T-wave oversensing. ICD-related infections occurred in 3.0% of the patients. Moreover, high rates of lead malfunction (6.3% annually) occur after ICD implantation in BrS patients. At the same time, rates of appropriate ICD therapies are comparatively low for primary prevention (0.9% per year) or secondary prevention (2.5% per year).8,9
In view of the above, S-ICD systems have become a valid alternative for many patients, especially younger individuals who do not need a pacing function, as they offer a long expected device lifetime and consequently an inherently lower risk of complications, as they have no transvenous electrodes.10 They reduce the risk of peri- and post-implantation complications, such as pneumothorax, pericardial effusion, lead fractures and dislodgment, that are mainly due to the leads implanted in or on the heart in conventional transvenous systems. Some studies also argue that S-ICD devices decrease infection rates; Boersma et al. showed an incidence of infections requiring device removal of 2.4% (24 patients) over a mean 3.1-year follow-up.10 In an observational study, Liang et al. concluded that S-ICDs are associated with a lower risk of endovascular infection, and thus have advantages for younger patients or those at high risk of infection.11 However, the literature is not consensual and some meta-analyses did not show superiority of S-ICDs in decreasing infection rates.12
Nonetheless, S-ICDs also have some drawbacks. Similarly to transvenous systems, one of the device-related complications of the S-ICD is inappropriate shocks due to T-wave oversensing, which is responsible for 73% of cases.9,13
Early publications of the EFFORTLESS S-ICD registry reported a 360-day inappropriate shock rate of 7% for the first-generation S-ICD (SQ-RXVR 1010; Cameron Health).6 Likewise, early major studies reported an overall incidence of inappropriate shocks of 13.1% over a mean 11-month follow-up14 and inappropriate shocks in 13% of patients, who received a total of 33 inappropriate shocks during an 18-month follow-up.15
Brower et al. found no statistical difference between inappropriate shock rates in subcutaneous versus transvenous ICD systems.16
Contemporary studies have reported lower inappropriate shock rates, of 3.5% per year17 and 11.7% over a mean follow-up of 3.1 years.10
The above data on inappropriate shocks precede implementation of the SMART Pass filter within the INSIGHT algorithm in the third-generation S-ICD (Emblem™ A219; Boston Scientific) and the AST. Theuns et al. showed that the SMART Pass filter reduced inappropriate shocks due to cardiac oversensing (1.6% with SMART Pass enabled vs. 6.4% with it disabled; p<0.001).18
Studies that support the same reductions with AST are lacking. Nevertheless, we consider that the use of AST is an advantage of our study, since, in contrast to the previous manual screening tool, which exhibits considerable interobserver variability, the novel AST is more tolerant of large T-waves and provides more consistent outcomes by removing operator subjectivity.19
In order to avoid T-wave oversensing and consequently inappropriate shocks, thorough assessment of patient eligibility prior to S-ICD placement is required, especially in BrS patients.
According to Conte et al., patients with BrS presented a higher rate of screening failure than those with other channelopathies (18% vs. 5%, p=0.07).13 This figure is similar to that found in our study, in which 14.3% of patients were considered ineligible before EST.
The same study showed that among patients with channelopathies, those with BrS exhibit significantly lower rates of suitable sensing vectors, which is also compatible with our study, in which 48% of patients who underwent EST had fewer eligible vectors than in baseline screening.
As in our study, Tachibana et al. found a higher prevalence of CRBBB in ineligible than in eligible BrS patients. To avoid inappropriate cardioversion, morphological changes on the standard 12-lead electrocardiogram can provide initial clues to physicians to reconsider whether patients will be eligible for an S-ICD. In particular, intermittent CRBBB is sometimes observed in BrS, and could be associated with inappropriate therapies using an S-ICD.2
It is also relevant that the typical BrS pattern is not static but dynamic, and may vary according to body temperature, vagal tone, or exercise.19 Kawabata et al. demonstrated that in some BrS patients S-ICD eligibility fluctuated between appropriate and inappropriate due to variation in ECG morphology. Therefore, multiple assessments of S-ICD appropriateness are recommended in BrS patients to ensure that ECG vector changes do not lead to inappropriate shocks.20
Exercise plays a fundamental role in ECG alterations and in triggering T-wave oversensing. Kamakura et al. showed that T-wave oversensing occurred during exercise in 33% of BrS patients in the vectors judged as optimal at the pre-implant ECG screening.5 This may explain the higher frequency of ineligible vectors in younger patients who achieved a higher heart rate seen in our study, and may have a greater impact on screening after EST in larger samples.
Sinus tachycardia during exercise attenuates ST elevation, but vagal activation during the recovery phase increases ST elevation and unmasks type 1 ECG pattern in patients with BrS. Makimoto et al. demonstrated augmentation of ST-segment elevation during the recovery phase of stress testing in 37% of BrS patients; this change was associated with VF events19 and can also change patient eligibility during screening after EST.
In our study, 85% of BrS patients were eligible according to the AST at rest, but 16.7% of those who passed the screening at rest became ineligible during EST. These figures are comparable to those reported by Tachibana et al.,2 supporting the idea that EST should be carried out to improve the sensitivity of the S-ICD eligibility test.
Another possible approach to provoke individual BrS morphology is by pharmacological application of Na+ channel blockers. In a study by Olde Nordkam et al., 21 patients who were eligible for S-ICD implantation at rest underwent ajmaline testing and developed a type 1 Brugada phenotype, which led to ineligibility for S-ICD implantation in five of these 21 patients.21 Ajmaline challenge unmasks screening failure in up to 15% of drug-induced BrS patients previously considered suitable for S-ICD implantation.13
In the present study, we opted for EST instead of a drug challenge test because exercise tests simulate everyday behavior, and may thus be better suited to identifying patients in whom S-ICD therapy may be inappropriate. In addition, exercise tests are easier for outpatients to perform, because follow-up ECG monitoring for hours after the stress test is not necessary, whereas drug challenge tests may sometimes induce VA even several hours after testing, until the drug is washed out from the body.
Finally, to the best of our knowledge, this is the first study conducted in Europe to assess eligibility after EST, showing that a significant number of patients with BrS become ineligible after EST, and consequently that it is important to perform EST before S-ICD implantation.
LimitationsThe main limitations of this study are the sample size and consequently the small absolute number of patients with negative screening, which limits its ability to identify predictors. Additionally, the study only analyzes eligibility based on ECG screening, without analyzing other factors such as the need for antibradycardia or antitachycardia pacing, and only the left-side lead was tested. The sample also includes patients with and without indication for ICD, and therefore includes patients with highly variable phenotypic expressions of the disease. Lastly, only a single center was included. Multicenter studies including larger numbers of patients need to be conducted to obtain more solid evidence.
ConclusionS-ICDs may be a valuable option for patients with indication for ICD as they are associated with lower rates of complications than transvenous systems.
In our sample, 28.6% (n=10) of patients were ineligible for S-ICD placement. Half of the patients were ineligible only after EST, corresponding to 16.7% of previously eligible patients.
There was a statistically significant correlation between the reduction in number of vectors eligible for an S-ICD and young age and maximum HR in EST. On the standard 12-lead electrocardiogram, CRBBB correlated with failure to meet the indications for an S-ICD before EST.
These results demonstrate the importance of screening after EST in all patients with BrS and with indication for an S-ICD, and may influence decisions concerning which ICD to implant or whether to institute pharmacological measures that avoid cases of inappropriate therapies.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.