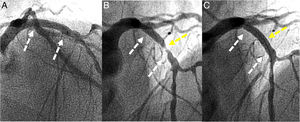
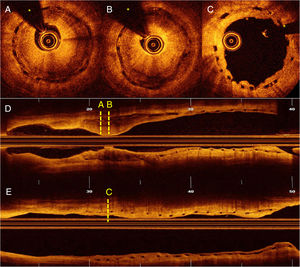
A 60-year-old man was admitted for unstable angina. Two-years ago he was treated with two bioresorbable vascular scaffolds (BVSs) on the proximal and distal segments of the left anterior descending coronary artery (Figure 1A, white arrows). Coronary angiography showed critical in-scaffold restenosis of the proximal BVS (Figure 1B, yellow arrow). Optical coherence tomography revealed heterogeneous tissue filling the BVS (Figure 2, A, B and D) with areas depicting bright neointimal hyperplasia with dorsal attenuation and marked shadowing of the underlying BVS struts. There was no evidence of scaffold disruption and neoatherosclerosis was considered the cause of late BVS failure. The patient was treated with a 3×15-mm sirolimus coated balloon catheter (drug-eluting balloon, DEB) (ratio of drug-eluting balloon to BVS size: 1:1) (MagicTouch™, Concept Medical Inc.) with excellent angiographic (Figure 1C) and optical coherence tomography (Figure 2, C and E) outcomes.
The unique properties of BVS, which provide the benefits of a powerful antiproliferative drug without the need for a permanent metal layer, make this device an attractive strategy during coronary interventions. Late BVS failure is a rare event and the treatment of choice for BVS restenosis has yet to be established.
Sirolimus-eluting balloons provide a novel, highly attractive therapy for patients with in-stent restenosis, but there are very limited data about the use of DEB in this context. Furthermore, the relative efficacy of this novel therapeutic modality, as compared with second-generation drug-eluting stents (DESs), has not yet been established. The use of DEB for BVS restenosis would avoid the need for an additional permanent metal layer (“leave nothing behind” strategy). This could be of particular value when excellent final outcomes are obtained, as in our case. To the best of our knowledge, this is the first report describing the use of sirolimus-eluting balloons for late BVS failure as a result of neoatherosclerosis.
Conflicts of interestThe authors have no conflicts of interest to declare.