Heart failure (HF) is a clinical syndrome associated with substantial morbidity, mortality, and healthcare costs. Dapagliflozin has proven efficacy in reducing the risk of death and hospitalization in HF patients, regardless of left ventricular ejection fraction (LVEF). This paper aimed to project the potential impact of dapagliflozin on healthcare costs related to HF subsequent hospitalizations (HFHs) in Portuguese hospitals.
MethodsThe total number of HF-related hospitalizations (hHF), HFHs, and the average length of stay for patients with a primary diagnosis of HF from six Portuguese hospitals, between January 2019 and December 2021, were collected and aggregated by hospital classification. Costs associated with HFHs were calculated according to Portuguese legislation and considering conservative, average, and complex approaches. Cost-saving projections were based on extrapolations from hHF risk reductions reported in dapagliflozin clinical trials.
ResultsConsidering a 26% risk reduction in hHF reported on pooled-analysis of DAPA-HF and DELIVER as the expected reduction in HFHs, the use of dapagliflozin would be associated with cost savings ranging from EUR 1612851.54 up to EUR 6587360.09, when considering all hospitals and the different approaches, between 2019 and 2021. A similar projection is observed based on 24% RRR derived by weighting DAPA-HF and DELIVER sub-analyses and PORTHOS epidemiological data.
ConclusionsIn this projection, dapagliflozin use in all eligible hHF patients is associated with a significant reduction in direct costs. Our data support that, in addition to the improvements in HF-related outcomes, dapagliflozin may have a significant economic impact on healthcare costs in Portuguese hospitals.
A insuficiência cardíaca (IC) é uma síndrome clínica associada a uma elevada morbidade, mortalidade e despesa com custos de saúde. A dapagliflozina demonstrou ser eficaz na redução do risco de morte e hospitalização em doentes com IC, independentemente da fração de ejeção do ventrículo esquerdo. Este artigo visa projetar o potencial impacto da dapagliflozina na redução dos custos relacionados com hospitalizações subsequentes por IC em hospitais portugueses.
MétodosFoi recolhido, e posteriormente analisado de forma agregada em clusters, o número total de hospitalizações por IC, hospitalizações subsequentes por IC e duração média de internamento dos doentes com diagnóstico primário de IC em seis hospitais portugueses, entre janeiro de 2019 e dezembro de 2021. Os custos relacionados com hospitalizações subsequentes por IC foram calculados de acordo com a legislação portuguesa e tendo em consideração três abordagens – conservadora, média e complexa. A projeção da redução de custos teve por base as reduções do risco relativo (RRR) de hospitalizações por IC (hIC) reportadas nos ensaios clínicos com dapagliflozina.
ResultadosConsiderando uma RRR de 26% do risco de hospitalização subsequente por IC, com base no valor reportado na análise agregada dos estudos DAPA-HF e DELIVER relativa à RRR de hIC, a nossa projeção indica que a utilização da dapagliflozina resulta numa redução de custos. De acordo com a nossa projeção a redução de custos oscila entre €1,612,851.54 e €6,587,360.09, entre 2019 e 2021, ao considerar todos os hospitais envolvidos e as diferentes abordagens metodológicas. A projeção aponta para um resultado similar tendo por base uma RRR de 24%, resultante da ponderação das subanálises de DAPA-HF e DELIVER, juntamente com dados epidemiológicos do estudo PORTHOS.
ConclusõesNesta projeção, a utilização de dapagliflozina, em todos os doentes hospitalizados por IC que sejam elegíveis, está associada a uma redução direta significativa nos custos hospitalares. Estes dados reforçam a ideia de que, para além da melhoria nos resultados de saúde relacionados com a IC, a dapagliflozina pode ter um impacto significativo nos hospitais portugueses ao nível dos custos de saúde.
Heart failure (HF) stands as a pressing public health issue, exerting a substantial negative impact on healthcare costs and quality of life for those affected.1–3 HF affects more than 64 million people worldwide, including 15 million in Europe.1,4,5 The Heart Failure Association Atlas 2019 report showed a median HF incidence in Europe of three per 1000 person-years for all ages and a median prevalence of 17 cases per 1000 people, while data from the EPICA study, published in 2002, reported a prevalence of 43.6 cases per 1000 people.2,6 Prevalence is expected to increase with an aging population, in addition to the extended survival observed in patients following guideline-directed medical therapy (GDMT).7–9 Recently, in Portugal, data from the PORTHOS observational study were presented showing an HF prevalence of 16.54% in the population aged 50 years or more, according to an updated and guideline-based diagnosis approach (Rui Baptista and Cristina Gavina, personal communication, December 12, 2023).
Heart failure has a major economic impact and implies high direct and indirect costs.10 Hospital care, due to the high rate of hospitalizations, is the major driver of HF costs.10 Not only does HF represent a significant economic burden to the healthcare systems, accounting for 1–3% of global health expenses, but the costs associated with in-hospital treatment are estimated to represent 39–75% of the total HF costs.3,11 More than one million hospitalizations deriving from HF have been reported in Europe and America per year, as well as readmission rates of 30% and multiple readmissions.1,12 Following an HF diagnosis, patients are hospitalized, on average, once per year.13 Globally, the number of hospitalizations may be expected to increase by up to 50% in the next 25 years, due to the aging population and the increased prevalence of comorbidities.14,15 In Portugal, 39% of the costs associated with HF derive from hospitalizations.3 As HF prevalence in Portugal is expected to increase by 30% by 2035, the total costs are also predicted to rise to EUR 503 million during the next decade.3 Simultaneously, a rise in the in-hospital mortality can be expected.16 Effective treatments that allow a reduction in HF-related hospitalizations (hHF) can play a significant role in the reduction of long-term HF costs.3
Several successful HF therapies have been developed in recent years. Among them, SGLT2 inhibitors (SGLT2i), including dapagliflozin, and empagliflozin, have been shown to reduce the risk of cardiovascular death and hospitalization in HF patients.17,18 First, SGLT2i have proven their efficacy in patients with HF and reduced ejection fraction (HFrEF).19,20 In 2021, the results of the SOLOIST-WHF trial showed the benefits of sotagliflozin, a dual SGLT2 and SGLT1 inhibitor, in reducing deaths from cardiovascular causes, hospitalizations, and urgent visits for HF in patients with diabetes and recent worsening HF, regardless of EF.21 Then, the EMPEROR-Preserved trial and DELIVER showed the benefit of SGLT2i in HF patients with mildly reduced (HfmrEF) and preserved ejection fraction (HfpEF), regardless of type 2 diabetes.22,23 Additionally, the DELIVER study showed benefits for patients who were hospitalized or had been recently hospitalized with HF, and for those with HF with improved LVEF.23,24
Both the European Society of Cardiology (ESC) and the American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guidelines recommend SGLT2i use in the treatment of patients with HFrEF, regardless of diabetes status (class of recommendation I, level of evidence A) due to the beneficial role in decreasing hHF and mortality.25,26 SGLT2i are also recommended for the treatment of patients with both HfmrEF and HfpEF (class of recommendation I, level of evidence A and class of recommendation II, level of evidence B, according to the ESC and AHA/ACC/HFSA guidelines, respectively).25,27
The benefit of SGLT2i across all HF patients, regardless of LVEF, represents a significant breakthrough, particularly for patients with HfpEF who had no previously effective therapeutic options except for control of comorbidities and symptomatic relief.22,23 Given the high prevalence of HfpEF, the opportunity SGLT2i bring is considerable. Therefore, considering the economic burden of HF and the robust evidence that consolidates the unique role of SGLT2i, we aimed to gather insights on the impact dapagliflozin might have on cost outcomes had it been used in a hospital setting. For this purpose, we hypothesized that the use of in-hospital dapagliflozin to all eligible patients will be associated with a decrease in healthcare costs associated with HF subsequent hospitalizations (HFHs) in six Portuguese hospitals.
ObjectivesUsing a modeling approach that was informed by clinical study results, epidemiological data, and real-world evidence data, the aim of this paper was to project the economic impact of dapagliflozin on HFHs when started on patients who were hospitalized for HF.
MethodsPopulationFor this economic projection, we gathered information from six Portuguese hospitals and included patients hospitalized with a primary HF diagnosis between the years of 2019 and 2021 retrospectively. The HF diagnosis was coded in accordance with the International Classification of Diseases (ICD) ninth and tenth revisions (Tables S1 and S2). Considering the renal restrictions associated with dapagliflozin use (estimated glomerular filtration rate [eGFR] must be >25 mL/kg/1.73 m2 for treatment initiation), we adjusted the population eligible for dapagliflozin in this projection by considering data from renal function characterizations documented in an available Portuguese HF cohort.28 Specifically, to be conservative, we excluded 19% of coded HF patients due to a eGFR <30 mL/kg/1.73 m2, according to published data.28 For an additional projection, we excluded a further 44.7% of patients corresponding to HF patients with diabetes, as reported in the literature.29 The data collected were then aggregated and analyzed based on hospital clusters: university hospitals (2), non-university hospitals (2) and HF clinics (2).30 Additionally, we performed an independent analysis of dapagliflozin-associated cost reduction by using previously published HF Portuguese data.31
Assumptions for heart failure cost projectionThe estimated cost per day of HFHs was determined by calculating the mean in-patient daily cost (overnight costs) and hospitalization prices (maximum costs of hospitalization, influenced by other factors, such as comorbidities) in accordance with Portuguese legislation (Table S3).32 The cost projection was conducted using three approaches (conservative, average and complex), taking into consideration the costs associated with the four levels of HF severity (minor, moderate, major, or extreme)32 (Table S3). In the conservative approach, the cost associated with the lower level of severity for all HF patients was used, resulting in a HFHs cost per day of EUR 876.07. The average approach was estimated by calculating the average of the cost values associated with the four levels of severity, amounting to EUR 1859.98 per day. Finally, the complex approach used the cost value associated with the highest level of severity (extreme), totaling EUR 3568.44 per day (Table S4).32
Concerning medication costs, only dapagliflozin treatment was considered, excluding other interventions (drugs or devices). Based on the assumptions that other treatments were used equally independently of dapagliflozin, excluding costs of other treatment would not impact the cost reduction associated with dapagliflozin.
Clinical inputs regarding HFHs cost-saving projectionFor the projection of HFHs-related savings associated with dapagliflozin use in hHF patients, we utilized the collected aggregated data by clusters of hospitals and the dapagliflozin reported outcomes in clinical trials as inputs to our projection model. We projected two scenarios on HFHs cost reduction by extrapolating expected risk reductions on HFHs based on the hHF relative risk reductions obtained in the DAPA-HF and DELIVER clinical trials.19,23 Scenario A considered a 26% expected reduction on HFHs. In the DAPA-HF and DELIVER pooled-analysis the reported relative risk associated with dapagliflozin for a first hHF and total HF hospitalizations were 26% and 29%, respectively. Therefore, to be cautious, we considered the value reported for the first hHF as an extrapolation for the projection of HFHs in our model, given that the reduction in HFHs-associated risk in the DAPA-HF and DELIVER pooled-analysis likely falls >29%. The scenario B considered an expected HFHs extrapolated from the 36% hHF relative risk reduction in HFrEF patients with a previous hospitalization in less than one year, and a 24% risk reduction in both HfmrEF and HfpEF patients that were recently hospitalized.33,34 Since the data collected from the Portuguese hospitals did not differentiate among the HF phenotypes, we used the recent data from PORTHOS study to weight the values extrapolated from the literature according to PORTHOS HF phenotype reported prevalence. Thus, since PORTHOS reported a prevalence of 0.35%, 0.97% and 15.22% for HFrEF, HfmrEF and HfpEF in mainland Portugal (Rui Baptista and Cristina Gavina, personal communication, December 12, 2023), we considered a reduction of 24% of hHF risk, according with the formula: % risk reduction=(0.36×0.02)+(0.24×0.98), where the 2% and 98% are the proportion of Portuguese HF patients with either HFrEF or HfmrEF and HfpEF, respectively.
For projections, we used the collected data and calculated the costs associated with patient subsequent hospitalizations using the values described above. The details of projections are illustrated in Table S5.
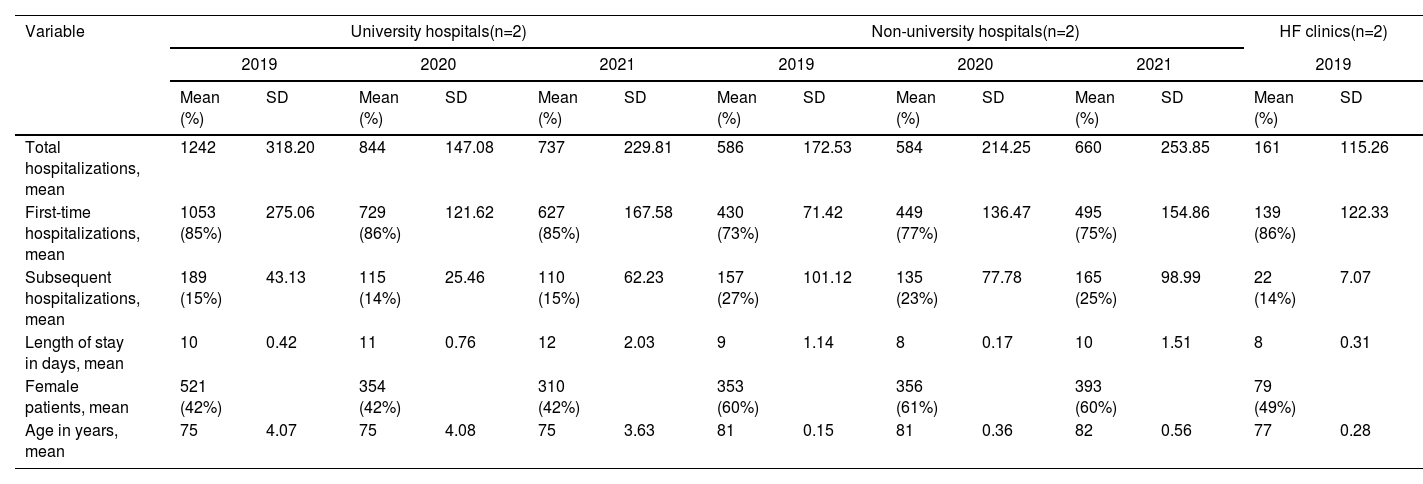
ResultsHospitalization and patient characteristicsBetween January 2019 until December 2021, a total of 4813 hHF were registered in the enrolled hospitals. Among these, 81.5% corresponded to first-time hospitalizations, and 18.5% to HFHs. The average length of stay for readmissions was 10 days. Out of the total of hHF, 58.6% were registered at university hospitals, 38.0% in non-university hospitals, and only 3.4% in HF clinics. However, data from HF clinics were only collected for the year 2019 due to COVID-19 constraints.
Regarding patient's characteristics, 49.2% were female. The mean age of hHF patients was 78 years. Detailed information on the number of hospitalizations, average number of hospitalization days and patient's characteristics are expressed in Table 1.
Characteristics of heart failure admissions in study hospitals.
| Variable | University hospitals(n=2) | Non-university hospitals(n=2) | HF clinics(n=2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | ||||||||
| Mean (%) | SD | Mean (%) | SD | Mean (%) | SD | Mean (%) | SD | Mean (%) | SD | Mean (%) | SD | Mean (%) | SD | |
| Total hospitalizations, mean | 1242 | 318.20 | 844 | 147.08 | 737 | 229.81 | 586 | 172.53 | 584 | 214.25 | 660 | 253.85 | 161 | 115.26 |
| First-time hospitalizations, mean | 1053 (85%) | 275.06 | 729 (86%) | 121.62 | 627 (85%) | 167.58 | 430 (73%) | 71.42 | 449 (77%) | 136.47 | 495 (75%) | 154.86 | 139 (86%) | 122.33 |
| Subsequent hospitalizations, mean | 189 (15%) | 43.13 | 115 (14%) | 25.46 | 110 (15%) | 62.23 | 157 (27%) | 101.12 | 135 (23%) | 77.78 | 165 (25%) | 98.99 | 22 (14%) | 7.07 |
| Length of stay in days, mean | 10 | 0.42 | 11 | 0.76 | 12 | 2.03 | 9 | 1.14 | 8 | 0.17 | 10 | 1.51 | 8 | 0.31 |
| Female patients, mean | 521 (42%) | 354 (42%) | 310 (42%) | 353 (60%) | 356 (61%) | 393 (60%) | 79 (49%) | |||||||
| Age in years, mean | 75 | 4.07 | 75 | 4.08 | 75 | 3.63 | 81 | 0.15 | 81 | 0.36 | 82 | 0.56 | 77 | 0.28 |
HF: heart failure; SD: standard deviation.
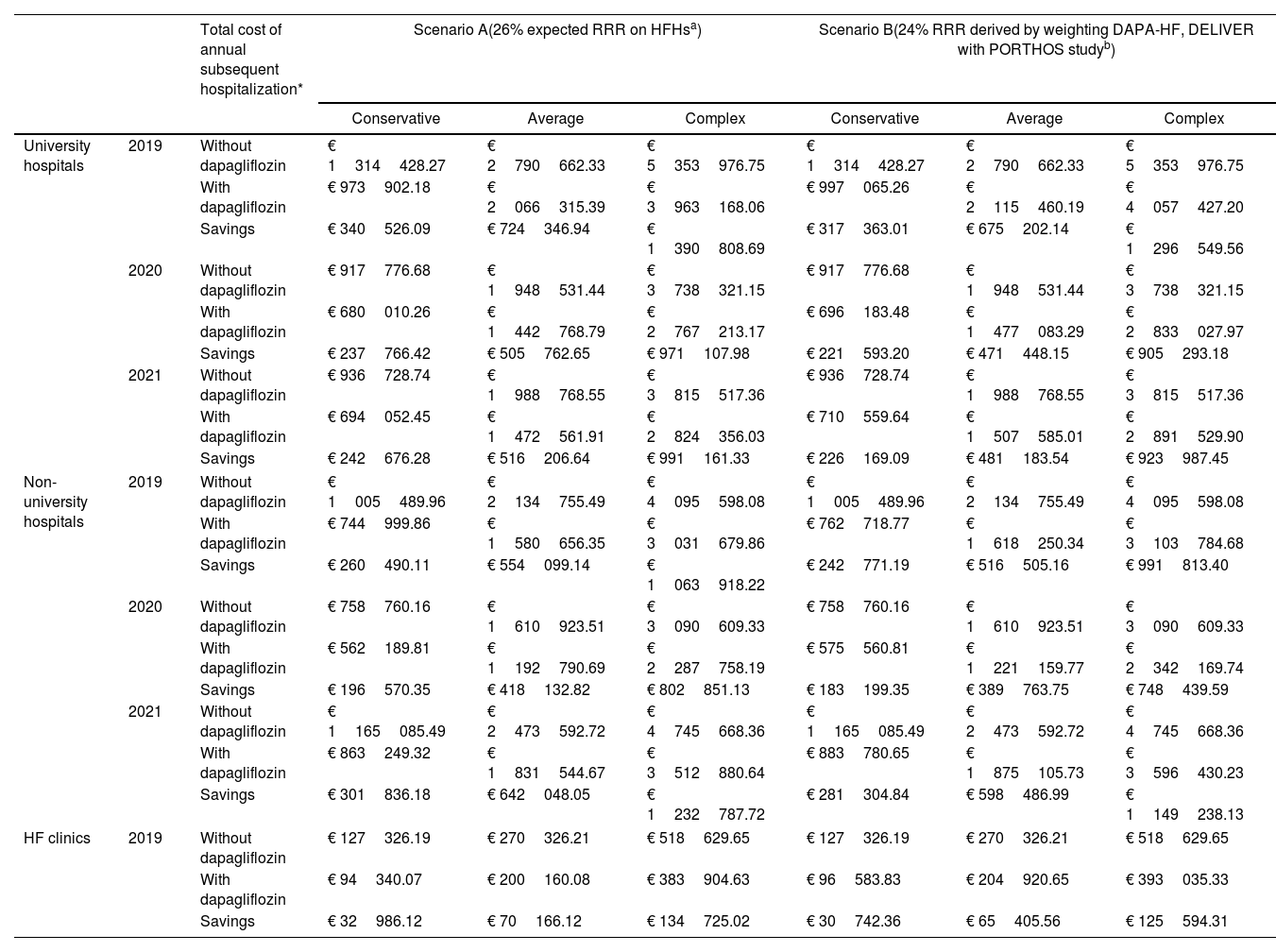
The detailed costs associated with HFHs in the different hospital clusters, years, and approaches, as well as the savings associated with dapagliflozin using the two scenarios, are presented in Table 2. In scenario A we projected HFHs cost savings associated with dapagliflozin use, considering a 26% reduction in HFHs. At university hospitals, 2019 and 2021, dapagliflozin initiation was associated with a reduction of EUR 820968.79, EUR 1746316.23, and EUR 3353078.00 in the conservative, average and complex approaches, respectively (Table 2). Similarly, projections applied to the non-university hospitals showed reductions in HFHs costs of EUR 758896.63, for the conservative approach; EUR 1614280.02 in the average approach; and EUR 3099557.07 for the complex approach, in the same years (Table 2). In 2019, the implementation of dapagliflozin in HF clinics was associated with reductions of EUR 32986.12, EUR 70166.12, and EUR 134725.02 considering the conservative, average and complex approaches, respectively (Table 2). According to our model, cost savings associated with the implementation of dapagliflozin for all eligible hHF patients could range from EUR 1612852 in the conservative approach, to EUR 6587360 in the complex approach.
Cost-saving projections for hospital readmissions associated with dapagliflozin use.
| Total cost of annual subsequent hospitalization* | Scenario A(26% expected RRR on HFHsa) | Scenario B(24% RRR derived by weighting DAPA-HF, DELIVER with PORTHOS studyb) | ||||||
|---|---|---|---|---|---|---|---|---|
| Conservative | Average | Complex | Conservative | Average | Complex | |||
| University hospitals | 2019 | Without dapagliflozin | € 1314428.27 | € 2790662.33 | € 5353976.75 | € 1314428.27 | € 2790662.33 | € 5353976.75 |
| With dapagliflozin | € 973902.18 | € 2066315.39 | € 3963168.06 | € 997065.26 | € 2115460.19 | € 4057427.20 | ||
| Savings | € 340526.09 | € 724346.94 | € 1390808.69 | € 317363.01 | € 675202.14 | € 1296549.56 | ||
| 2020 | Without dapagliflozin | € 917776.68 | € 1948531.44 | € 3738321.15 | € 917776.68 | € 1948531.44 | € 3738321.15 | |
| With dapagliflozin | € 680010.26 | € 1442768.79 | € 2767213.17 | € 696183.48 | € 1477083.29 | € 2833027.97 | ||
| Savings | € 237766.42 | € 505762.65 | € 971107.98 | € 221593.20 | € 471448.15 | € 905293.18 | ||
| 2021 | Without dapagliflozin | € 936728.74 | € 1988768.55 | € 3815517.36 | € 936728.74 | € 1988768.55 | € 3815517.36 | |
| With dapagliflozin | € 694052.45 | € 1472561.91 | € 2824356.03 | € 710559.64 | € 1507585.01 | € 2891529.90 | ||
| Savings | € 242676.28 | € 516206.64 | € 991161.33 | € 226169.09 | € 481183.54 | € 923987.45 | ||
| Non-university hospitals | 2019 | Without dapagliflozin | € 1005489.96 | € 2134755.49 | € 4095598.08 | € 1005489.96 | € 2134755.49 | € 4095598.08 |
| With dapagliflozin | € 744999.86 | € 1580656.35 | € 3031679.86 | € 762718.77 | € 1618250.34 | € 3103784.68 | ||
| Savings | € 260490.11 | € 554099.14 | € 1063918.22 | € 242771.19 | € 516505.16 | € 991813.40 | ||
| 2020 | Without dapagliflozin | € 758760.16 | € 1610923.51 | € 3090609.33 | € 758760.16 | € 1610923.51 | € 3090609.33 | |
| With dapagliflozin | € 562189.81 | € 1192790.69 | € 2287758.19 | € 575560.81 | € 1221159.77 | € 2342169.74 | ||
| Savings | € 196570.35 | € 418132.82 | € 802851.13 | € 183199.35 | € 389763.75 | € 748439.59 | ||
| 2021 | Without dapagliflozin | € 1165085.49 | € 2473592.72 | € 4745668.36 | € 1165085.49 | € 2473592.72 | € 4745668.36 | |
| With dapagliflozin | € 863249.32 | € 1831544.67 | € 3512880.64 | € 883780.65 | € 1875105.73 | € 3596430.23 | ||
| Savings | € 301836.18 | € 642048.05 | € 1232787.72 | € 281304.84 | € 598486.99 | € 1149238.13 | ||
| HF clinics | 2019 | Without dapagliflozin | € 127326.19 | € 270326.21 | € 518629.65 | € 127326.19 | € 270326.21 | € 518629.65 |
| With dapagliflozin | € 94340.07 | € 200160.08 | € 383904.63 | € 96583.83 | € 204920.65 | € 393035.33 | ||
| Savings | € 32986.12 | € 70166.12 | € 134725.02 | € 30742.36 | € 65405.56 | € 125594.31 | ||
HF: heart failure; HFHs: heat failure subsequent hospitalizations; RRR: relative risk reduction.
Total cost of annual subsequent hospitalizations in patients that could have initiated dapagliflozin during the index-hospitalization due to heart failure.
The scenario A considered a 26% expected RRR on HFHs. In the DAPA-HF and DELIVER pooled-analysis the reported RR associated with dapagliflozin for a first hHF and total HF hospitalizations were 26% and 29%, respectively. Therefore, to be cautious, we considered the value reported for the first hHF as an extrapolation for the projection of HFHs in our model, given that the reduction in HFHs-associated risk in the DAPA-HF and DELIVER pooled-analysis likely falls above 29%.
In scenario B, in the conservative approach, we observed cost reductions of EUR 765125.30 for the university hospitals between 2019 and 2021. In the average approach, the cost savings were EUR 1627833.84 for the same years. In the complex approach, we observed a cost reduction of EUR 3125830.19 (Table 2). Applying this second projection to data from non-university hospitals, we observed a reduction in HFHs costs of EUR 707275.38, EUR 1504755.90, and 2889491.11 in the same years, considering the conservative, average and complex approaches, respectively (Table 2). Finally, in HF clinics, the cost savings associated with dapagliflozin considering a 24% reduction of HFHs risk were EUR 30742.36 in the conservative approach, EUR 65405.56 in the average approach, and EUR 125594.31 in the complex approach (Table 2). Overall, in scenario B the use of dapagliflozin, and taking into account all years in the study this projection indicates a cost reduction up to EUR 6140916.
SGLT2i were already widely used in HF diabetic patients, therefore, a significant proportion of patients in our analysis could have previously taken or started an SGLT2i, which has a proven impact on HFHs. To address this limitation, we performed an independent analysis excluding 44.7% of patients corresponding to the reported diabetes incidence among HF patients.29 Even excluding these patients, cost savings associated with dapagliflozin implementation can reach EUR 718501.50, EUR 1528353.89, and EUR 2934571.47 in the conservative, average and complex approaches, respectively, when considering the 26% HFHs risk reduction for all the years and hospitals in the study (Table S6). Taking into account the 24% HFHs risk reduction, cost savings range between EUR 669627.99, EUR 1424659.60 and EUR 2735687.07 in the conservative, average, and complex approaches for all hospitals and years analyzed (Table S6).
Cost saving associated with dapagliflozin use in previous published Portuguese heart failure cohortsAccording to the literature, between 2012 and 2014, 51310 patients with a primary diagnosis of HF were hospitalized in Portuguese hospitals.31 From these, 22.1% were considered heavy users, meaning that they had two or more HF admissions within a 365-day period.31 According to the same study, the length of hospital stay for frequent users was at least 20 days.31 Taking into consideration these data, according to scenario A, dapagliflozin could have saved EUR 41486961.55, EUR 88248610 or EUR 169444954 considering the conservative, average, and complex approaches, respectively between 2012 and 2014. If we consider scenario B cost savings reach the EUR 38664958.16, EUR 82261202.84, and EUR 157961178.47 in each approach.
DiscussionHeart failure is related to significant morbidity and mortality, representing an important social and economic burden.35 The increasing aging of the population, and the improvement in healthcare including innovative secondary prevention therapy and rehabilitation programs, are factors that highly contribute to the rising incidence and prevalence of this condition.3 According to the literature, HF costs represent 2.6% of total Portuguese public health expenditure.3 Furthermore, costs related to HF admissions represent 1.6% of Portuguese hospitals’ budgets, and over 41% of these costs are attributed to patients that are re-hospitalized at least once within one year after previous discharge.31 Given this approach, the approval of therapies that significantly reduce hHF risk can have an important impact on healthcare costs.
Dapagliflozin was associated with a reduction in the number of HF hospitalizations and subsequent hospitalizations.33,34,36 In this paper, we projected the impact of in-hospital dapagliflozin use on healthcare costs related to HF readmissions by analyzing aggregated data collected from different Portuguese hospitals. The complexity of HF management in an hHF setting varies significantly, influenced by factors such as whether it is the first or a repeated event, comorbidities, the severity of the condition, the type of hospitalization and medical specialty. Therefore, for simplification, in this projection, the data were aggregated into three different approaches. Although the data used for the projection may not be representative of the national reality, this approach aimed to create a simple and comprehensive approach that reflects the economic impact of dapagliflozin use in all eligible patients in the different healthcare infrastructures (in terms of size) where an HF patient can be managed. Categorization of the data collected by the hospital sources provides a snapshot of these savings in these three different realities. We also evaluated data from HF clinics. Although these clinics can be placed in university and non-university hospitals, we also aimed to evaluate the impact of dapagliflozin in this setting since it is an increasing practice for patient management in our country. Our findings demonstrate that the use of in-hospital dapagliflozin for all eligible HF patients is associated with a significant decrease in costs in all considered approaches. Our projection model is based on the reported reduction of hHF risk associated with dapagliflozin use. Specifically, we project the HFHs cost savings associated with dapagliflozin use based on extrapolations for expected HFHs relative reductions based on data available from DAPA-HF and DELIVER clinical trials.33,34 Both DAPA-HF and DELIVER studies were multinational, double-blinded, placebo-controlled randomized clinical trials in patients with HFrEF and with mildly reduced, preserved, or improved LVEF, respectively. These studies evaluated the efficacy and safety of 10 mg dapagliflozin, daily, in comparison with placebo over a follow-up of 18.2 months (DAPA-HF) and 2.3 years (DELIVER).19,23
In scenario A, dapagliflozin was associated with HFHs cost savings ranging from EUR 32986.12, when applied to HF clinics in 2019, considering a conservative approach, to EUR 1390808.69, when considering the complex approach with data collected from 2019 in university hospitals. Similarly, if we consider the scenario B, cost savings can reach EUR 1296549.56 in the case of university hospitals in 2019. For these projections, we specifically focused on the medication expenses attributed to dapagliflozin only. This decision stemmed from our assumption that the utilization of other HF medications would likely maintain a similar rate, irrespective of dapagliflozin use. Moreover, our analysis presumed that the benefits associated with dapagliflozin remain constant regardless of variations in other patterns of HF treatment, as previously reported in the literature.37,38
In the university hospitals cluster, the use of dapagliflozin in the represented years could involve savings of EUR 813916.92 per hospital in an average approach. Considering that Portugal has seven university hospitals, savings could reach EUR 5697418.43 in these years.39 Additionally, for non-university hospitals, extrapolation we only considered hospitals equipped a cardiology unit. We estimate an average cost reduction of EUR 752377.95 per hospital resulting in a potential total cost reduction of EUR 22571338.43 for all the 30 Portuguese non-university hospitals with a structured cardiology unit.39 Notwithstanding, it is relevant to highlight that many HF patients are managed by internal medicine, and the role of internal medicine is paramount in the management of patients with acute HF. For simplification purposes, we took into account only these 30 hospitals, however even hospitals without a structured cardiology unit might benefit from cost savings associated with dapagliflozin use. Moreover, non-university hospitals vary in size, resources, and population served, and thus this value may be biased; the projection indicates the economic impact that dapagliflozin can have among Portuguese hospitals.
Regarding the HF clinics, although they have grown significantly internationally and even nationally in recent years, there is no information available on the exact number of HF clinics in our country. Therefore, we are unable to estimate the total savings that the use of dapagliflozin can generate in this context. Nevertheless, from the independent projection, our model pointed to potential significant savings. Overall, according to scenario B, Portuguese healthcare costs could be reduced in EUR 28268756.86 through the treatment with dapagliflozin to all eligible patients. It is also important to notice that even after excluding a significant percentage of patients corresponding to the HF diabetic population already treated with SGLT2i, our projections still indicate a significant cost saving associated with dapagliflozin use.
Although we did not intend it to be a cost-effectiveness study, the results from our projections are aligned with previous cost-effectiveness studies, in which dapagliflozin was a cost-effective treatment for HFrEF relative to GDMT when evaluated in various healthcare systems, including the US,40 UK, German, and Spain.41 A similar conclusion is indicated by recent cost-effectiveness studies of dapagliflozin in HfmrEF and HfpEF patients.42,43
In this projection, we considered data from HF readmissions coded as the main diagnosis, opting for conservative yet more accurate projection. Nevertheless, the cost savings could potentially be higher in clinical practice, when considering all hHF, given that hHF is often recorded as a secondary cause of readmission. Additionally, we focused only on the direct cost of patients’ readmissions to estimate cost savings. However, HF also represents a burden on patients’ quality of life, impacting the daily living activities of patients and their caregivers.10 Given the impact of dapagliflozin in improving HF-related outcomes, its implementation can also contribute to improvements in-patients’ quality of life.
We also applied our methodology to calculate the savings associated with hHF according to the Portuguese published data. Between 2012 and 2014, 51310 patients with a main diagnosis of HF have been hospitalized in Portuguese hospitals,31 leading to 68565 admissions and 665000 bed-days. The inpatient hospital services cost associated with these hospitalizations amounted to EUR 193 million, which corresponds to nearly 1.6% of the total costs from the national healthcare service.31 From the 51310 admitted patients, 22.1% had an average of 2.5 inpatient admissions per year, while 21.8% of these patients were re-hospitalized within 30 days, and 54.9% within 90 days of discharge.31 Considering this data and an average approach, the national health service could have saved up to EUR 82261202.84 if dapagliflozin had been available and implemented at that time.
On average, 19% of our sample HF patients were re-hospitalized at least once after a previous HF hospitalization. This aligns with published national results showing a subsequent hospitalizations rate of 22.10%.31 Our data are also consistent with the literature regarding the average age of patients (78 years for both) and gender distribution (51% female patients in our sample compared with 56% in published data).31 According to our data, the average length of stay for acute HF subsequent hospitalizations was approximately 10 days, which is lower than that reported in previous studies.31
LimitationsThis projection has several limitations that should be acknowledged. The clusters have only two sources which is not sufficient for a comprehensive representation of the entire cluster. Moreover, a broad standard deviation demonstrates the variability between the two sources. The data collected spanned three years, including the period of the coronavirus pandemic (COVID-19), which significantly affected access to healthcare and patient willingness to seek medical help. In Portugal, the first patient with COVID-19 was diagnosed in March 2020, so the 2019 data was not influenced by the pandemic. If HF hospitalization rates in 2020 and 2021 were similar to 2019, the cost savings would increase up to EUR 19762080.26 and EUR 18422746.86 considering 26% and 24% RRR on HFHs, respectively, in a complex scenario and for all considered hospitals. At the same time, we cannot rule out the worsening of HF COVID-19-related conditions.44 Therefore, our projection would benefit from the inclusion of data collected in recent years either before or after the pandemic. Additionally, data regarding the use of out-patient services were not assessed, and for simplicity, other not expected costs have a significant impact on the projections performed were also not included. Similarly, costs related with dapagliflozin side effects were not considered.
Another limitation concerns the public nature of the hospitals from which our data were collected, as the costs associated with patients’ follow-up in the private sector cannot be included, which could impact the reduction of health expenditure in the projections. Moreover, and as shown by previous studies focusing on the QoL of HF patients,36,45 dapagliflozin may help further reducing indirect costs associated to absenteeism related to consultations, emergency visits and hospitalizations. However, the evaluation of these costs falls outside the scope of this projection.
For this projection, we considered the recently presented data regarding HfrEF, HfmrEF and HfpEF incidence in Portugal (Rui Baptista and Cristina Gavina, personal communication, 12 December 2023). However, the characteristics of the inpatient population may not reflect this incidence since a higher number of hospitalizations due to HFrEF is expected when compared with the other forms of HF. For projection purposes, we assumed that all eligible HF patients initiated dapagliflozin treatment during their first hHF event each year (index hHF). However, it is plausible that some of these eligible patients might have been already receiving dapagliflozin treatment. To strengthen our conclusions, we performed a separate analysis excluding the percentage of reported HF diabetic patients. Furthermore, it is worth noting that dapagliflozin had received approval in Europe for HFrEF treatment, in addition to type 2 diabetes, by the end of 2020, and for HF treatment irrespective of LVEF in 2022. Considering the update of ESC HF guidelines occurred only in 2021, it is anticipated that the adoption rate of dapagliflozin treatment among non-diabetic HF patients from 2019 to 2021 remained relatively low.
Aside from the assumption mentioned above, our model has other important assumptions to be taken in to consideration in the discussion of the results. Firstly, we considered only HF readmissions occurring within the same year to calculate the subsequent hospitalizations rate; secondly, the mean length of hospital stays was used instead of the median (which would be less impacted by possible outliers); thirdly, the calculated costs used were based on the Portuguese legislation and not in data specifically gathered from each hospital. Additionally, our data sources comprised only two hospitals for each group, contributing to increased standard deviation. However, despite these limitations, the results consistently indicate substantial cost reductions across all approaches tested within hospital settings.
ConclusionsHeart failure has a significant economic impact on healthcare costs, and this economic impact is expected to increase in the coming years. A considerable proportion of HF costs are attributed to hospitalizations, which are frequently recurrent. We had no intention of developing a comprehensive cost-effectiveness model, but this simplified projection based on real-world data acts as a significant addition to the translation of clinical trial data into clinical practice. This paper underscores the vital importance of implementing scientific knowledge to everyday medical practice. Our projections point to a significant cost reduction in all tested approaches, highlighting the importance of dapagliflozin use in an economic context. In addition to the reported value of dapagliflozin in improving HF outcomes, our projection clearly indicates a substantial cost reduction associated with subsequent HF hospitalizations when dapagliflozin is started during a HF hospitalization in all HF eligible patients.
FundingFinancial support for the preparation of this article was provided by AstraZeneca.
Conflict of interestsDB received speaker and consultant fees from AstraZeneca, Bayer, Bial, Bristol-Myers Squibb, Novartis, Pfizer, and Vifor Pharma. CF received speaker and consultant fees from AstraZeneca, Bayer, Bial, Boehringer-Ingelheim, CSL Vifor, Novartis, Novo Nordisk, Pfizer, Servier. RB received speaker and consultant fees from AstraZeneca, Bayer, Bial, Boehringer-Ingelheim, Novartis, Servier, and CSL Vifor. JP received speaker and consultant fees from AstraZeneca, Bayer, Bial, Boehringer-Ingelheim, GSK, Lilly, Merck, Novartis, Pfizer, Roche Diagnostics, Servier, and CSL Vifor. IM received speaker and consultant fees from AstraZeneca, Bayer, Bial, CSL Vifor, Daiichi-Sankyo, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Servier. SG received speaker and consultant fees from AstraZeneca, Bayer, Novartis, and Boehringer-Ingelheim. FF received speaker and consultant fees from AstraZeneca, Bayer, Novartis, Boehringer-Ingelheim and Novo Nordisk. JSC has received speaker and consultant fees, advisory board participation fees, or investigational grants from Abbott, AstraZeneca Pharmaceuticals, Bial, Boehringer-Ingelheim, Menarini, Merck Serono, Merck Sharp & Dohme, Novartis, Orion, Pfizer, Sanofi, Servier, and Vifor Pharma. ML and MA are employees of AstraZeneca Portugal (medical department). JS, RR and VL do not have any conflicts of interest to declare. None of the authors did receive any fee related to the preparation of this article.
The authors acknowledge writing assistance provided by Raquel Duarte PhD and Irina Duarte PhD from x2 – Science Solutions, funded by AstraZeneca.