Percutaneous balloon aortic valvuloplasty (BAV) has been limited by the risk of complications and restenosis. However, growing use of transcatheter aortic valve implantation (TAVI) has revived interest in this technique. We analyzed the current indications for BAV and outcomes in a single center.
MethodsAcute results and long-term outcomes were analyzed in a retrospective single-center registry of patients undergoing BAV between January 2013 and January 2016.
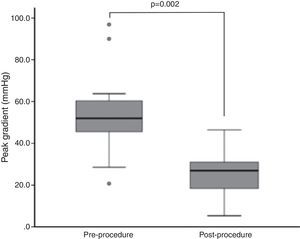
ResultsTwenty-three patients underwent BAV, 56.5% male, mean age 78±7 years. Indications were severe aortic stenosis and decompensated heart failure (n=5), urgent non-cardiac surgery (n=8), or bridge to definitive treatment (n=10). Peak invasive gradient decreased from a median of 54.0±19.0 mmHg to 28.5±13.8 mmHg (p=0.002). Complications included one ischemic stroke, one lower limb ischemia and one femoral pseudoaneurysm requiring surgery. During a mean follow-up of 11±10 months, eight patients underwent TAVI and two underwent surgical aortic valve replacement. Thirteen patients died, nine of non-cardiovascular causes. On Kaplan-Meier analysis mortality was significantly lower among patients undergoing definitive treatment (20.0% vs. 84.6% at two-year follow-up; p=0.005).
ConclusionBAV should be considered for selected patients with temporary contraindications to definitive therapy or as palliative therapy.
A implantação de próteses aórticas percutâneas reavivou o interesse na valvuloplastia aórtica por balão, habitualmente limitada por complicações e restenose. Analisámos as indicações e resultados desta técnica.
MétodosRegisto retrospetivo, unicêntrico, de doentes submetidos a valvuloplastia aórtica por balão, de janeiro de 2013 a janeiro de 2016. Analisaram-se os resultados imediatos e a longo prazo.
ResultadosVinte e três doentes foram submetidos a valvuloplastia aórtica por balão, 56,5% homens, idade média 78±7 anos. As indicações foram estenose aórtica grave com: insuficiência cardíaca descompensada (n=5); cirurgia não-cardíaca urgente (n=8); ponte para terapêutica definitiva (n=10). O gradiente de pico invasivo reduziu-se de uma mediana de 54,0 (19,0) mmHg para 28,5 (13,8) mmHg (p=0,002). Registaram-se um acidente vascular cerebral isquémico, uma isquemia aguda do membro inferior e um pseudoaneurisma femoral resolvidos cirurgicamente. Durante um seguimento médio de 11±10 meses, efetuaram-se oito implantações percutâneas de prótese aórtica e duas substituições cirúrgicas. Treze doentes morreram, nove de causas não-cardiovasculares. Por análise de sobrevivência de Kaplan-Meier, a mortalidade foi menor nos doentes submetidos a tratamento definitivo (20,0 versus 84,6% a dois anos; p=0,005).
ConclusãoA valvuloplastia aórtica por balão deve ser considerada em doentes selecionados com contraindicações temporárias ao tratamento definitivo ou como terapêutica paliativa.
Symptomatic severe aortic stenosis (AS) conveys a high risk of cardiovascular death and rehospitalization for heart failure with medical therapy alone. Without treatment mean survival is only 1-3 years after symptom onset.1 Surgical aortic valve replacement (SAVR) is the gold standard for the treatment of severe AS. However, mainly due to the high prevalence of comorbidities, up to one-quarter of patients do not undergo SAVR.2,3
For patients who are not suitable for surgical treatment, balloon aortic valvuloplasty (BAV) was first proposed in 1986 by Alain Cribier as a useful, low-risk, palliative treatment for symptomatic relief.4,5 Despite promising initial results (reduction of maximum and mean aortic gradients and improvement in functional capacity), its popularity waned, due to the high rate of complications and early restenosis. Moreover, the long-term survival of these patients is low, resembling the natural course of untreated severe AS.6
The introduction of transcatheter aortic valve implantation (TAVI) has revived interest in BAV for clinically unstable patients as a bridge to definitive therapy (TAVI or SAVR) or as a destination therapy for palliative reasons. Cohort B of the PARTNER trial showed that patients managed conservatively have significantly higher 12-month mortality compared to patients undergoing TAVI. However, only a small difference in six-month mortality was noted: 22% in the TAVI group vs. 28% in patients treated conservatively (of whom 83% underwent BAV).7 This good result in the first months supports BAV as a therapeutic bridge.
In the TAVI era, BAV is often performed to facilitate percutaneous delivery of the prosthesis, reduce paravalvular leaks and aid in ring size assessment. The growing number of BAV procedures, together with improvements in techniques and materials and use of vascular closure devices, has led to a reduction in procedural complications. While an older series had a 20% complication rate and 8% mortality, a more recent study reported much lower rates of major complications and overall mortality (6.8% and 2.5%, respectively).8,9
The aim of this study was to analyze the current indications for BAV and to determine the success, complication and survival rates after BAV of patients in a real-world setting.
MethodsThis was a retrospective single-center registry of patients undergoing BAV between January 2013 and January 2016.
Inclusion criteriaAll patients with symptomatic severe AS who underwent BAV were consecutively enrolled. The center's heart team assessed the indication for BAV.
Procedural indications were classified as: (1) bridge to recovery: refractory cardiogenic shock, pulmonary edema or congestive heart failure due to severe AS, including patients under invasive mechanical ventilation; (2) bridge to decision: patients in whom it was judged that LV systolic function might recover and clinical condition improve via BAV, enabling subsequent definitive treatment (TAVI or SAVR); and (3) bridge to non-cardiac surgery: patients in whom urgent non-cardiac surgery was needed but had a prohibitive risk due to untreated severe AS.
Data collectionBaseline clinical, laboratory, echocardiographic, hemodynamic and procedural data were collected retrospectively through reviews of hospital records. Follow-up was performed through hospital outpatient consultations or telephone interview. Laboratory and echocardiographic assessment was repeated three to six months after the procedure. Data on mortality were obtained from the National Patient Registry.
ProcedureBAV was performed under conscious sedation, with different balloon devices (Nucleus® and Loma Vista Medical®), by the standard retrograde technique and under rapid ventricular pacing. Regarding balloon diameter (which ranged from 20 to 25 mm), the choice was made according to the individual patient's characteristics. Some patients had already been assessed by cardiac computed tomography for TAVI, and so these measurements were used. In other cases, aortic valve diameter was measured by a combination of transthoracic echocardiogram (or, in ventilated patients, transesophageal echocardiogram) and fluoroscopic angiogram during the procedure. The access route was the right or left femoral artery (arterial sheaths ranging from 11 to 12F) and access site closure was performed in all cases using Perclose ProGlide™ (Abbott Vascular, Abbott Park, IL, USA) or Angio-Seal™ STS (St. Jude Medical, St. Paul, MN, USA) devices. An unfractionated heparin bolus was administered after sheath insertion (70-100 IU/kg).
Study endpointsThe primary endpoint was the incidence of all-cause death during long-term follow-up. The secondary endpoint was the composite of cardiovascular or unknown cause death and rehospitalization due to heart failure, stroke, or myocardial infarction. Serious adverse events were defined as stroke, coronary occlusion or dissection, moderate to severe aortic regurgitation, tamponade, permanent pacemaker requirement and vascular complication requiring intervention. All events were retrospectively adjudicated according to the second Valve Academic Research Consortium (VARC-2) statement.10
Statistical analysisThe statistical analysis was performed with IBM SPSS Statistics for Windows, version 20.0. Continuous values are reported as mean±standard deviation or median (interquartile range [IQR]), and categorical data are reported as number and percentage. The Wilcoxon test was used to compare two dependent nonparametric variables. Kaplan-Meier survival analysis was performed. Cox regression was used to identify independent predictors of mortality.
ResultsBaseline characteristics and demographicsBetween January 2013 and January 2016, 23 patients underwent standalone BAV procedures, 56.5% male, mean age 78±7 years. A total of 40 patients were included, of whom 34.8% were in New York Heart Association functional class IV (n=8), and the remainder were in class III. Baseline pre-implantation characteristics are summarized in Table 1. The risk of mortality according to the Society of Thoracic Surgeons (STS) score was 12.3±10.0% (with a risk of morbidity or mortality of 47.0±18.8%), and mean EuroScore II was 11.7±6.0.
Characteristics of the study population.
| Age, years (mean±SD) | 78±7 |
| Male, n (%) | 13 (56.5) |
| STS score (mean±SD) | 12.3±10.0 |
| EuroScore II (mean±SD) | 11.7±6.0 |
| NYHA functional class | |
| III (n, %) | 15 (65.2) |
| IV (n, %) | 8 (34.8) |
| Comorbidities | |
| CAD (n, %) | 12 (52.2) |
| Hypertension (n, %) | 17 (73.9) |
| AF (n, %) | 11 (52.2) |
| Dyslipidemia (n, %) | 12 (52.2) |
| Diabetes (n, %) | 10 (43.5) |
| CKD (n, %) | 10 (43.5) |
| COPD (n, %) | 4 (17.4) |
| Malignancy (n, %) | 6 (26.1) |
| Liver disease (n, %) | 2 (8.8) |
AF: atrial fibrillation; CAD: coronary artery disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons.
The main comorbidities were hypertension, coronary artery disease (CAD), dyslipidemia, atrial fibrillation (AF), diabetes, chronic kidney disease (CKD) (defined as Kidney Disease Outcomes Quality Initiative stage ≤3 [creatinine clearance <60 ml/min/1.73 m2]), chronic obstructive pulmonary disease (COPD) and malignancy (Table 1). A total of 26.9% of patients had previously undergone percutaneous coronary intervention (PCI) due to critical CAD.
Procedure detailsIndications for BAV were bridge to recovery (n=5), including three patients under invasive mechanical ventilation; bridge to non-cardiac surgery (n=8); and bridge to decision (n=10).
The pre-procedure maximum and mean aortic gradients measured by transthoracic echocardiogram were 71.7±24 mmHg and 42.9±13.3 mmHg, respectively, with aortic valve area of 0.7±0.3 cm2 (0.4±0.1 cm2/m2). Mean left ventricular ejection fraction (LVEF) was 40.2±17.4%, and mean pulmonary artery systolic pressure (PASP) was 53.7±13.5 mmHg.
The procedure was performed under conscious sedation, by femoral access, with 11-12 F sheaths. The Nucleus® balloon was used in the majority of cases (n=18; 78.3%), and the Loma Vista Medical® balloon in the remainder (n=5; 21.7%), with a mean balloon size of 20.8±1.5 mm. Two cases (8.9%) required two inflations. Vascular closure devices were used in all patients; the Perclose ProGlide™ was used in 91.3% of cases and the Angio-Seal™ STS in the remainder. Mean procedure duration was 77.0±29.3 min. Mean fluoroscopy time was 16.6±7.4 min, with a fluoroscopic radiation dose of 59.3±28.5 μGy/cm2 and a contrast dose of 71.1±66.3 ml.
Endpoints and outcomesThe peak gradient assessed invasively during the procedure decreased from a median of 54.0±19.0 mmHg to 28.5±13.8 mmHg (p=0.002) (Figure 1). Although without statistical significance, there was a trend for reductions in N-terminal pro-B-type natriuretic peptide (NT-proBNP) and PASP and improvement in LVEF during follow-up (NT-proBNP 4972±15401 pg/dl to 4654±8009 pg/dl, p=NS; PASP 53±18 mmHg to 39±11 mmHg, p=NS; LVEF 50±33% to 56±30%, p=NS).
There were three serious adverse events in two patients. One patient experienced an ischemic stroke and left lower limb ischemia requiring urgent surgery, and another a femoral pseudoaneurysm requiring elective repair. No other complications occurred, including acute moderate or severe aortic regurgitation, or need for urgent permanent pacemaker implantation, SAVR or TAVI.
All patients under invasive mechanical ventilation (n=3) were extubated soon after the intervention and patients undergoing non-cardiac surgery had no major perioperative cardiovascular complications.
MortalityThere was one BAV procedure-related death (4.3%) due to ischemic stroke and acute limb ischemia as procedural complications. Another patient had a right coronary artery rupture during PCI, and went into cardiogenic shock even after the rupture was resolved. He underwent BAV immediately, which resulted in some hemodynamic improvement, but eventually died in refractory cardiogenic shock. A third patient underwent a pre-TAVI left main PCI complicated by cardiogenic shock and BAV was performed successfully. Nevertheless, this patient also progressed to refractory cardiogenic shock and died. Overall in-hospital mortality was thus 13.0% (n=3).
During a mean follow-up of 258±303 days, 34.8% (n=8) of the patients underwent TAVI (mean time between BAV and TAVI 155.0±91.3 days) and 8.7% (n=2) underwent SAVR.
During follow-up, thirteen patients (56.5%) died, predominantly of non-cardiovascular causes (n=9, 69.2%), corresponding to the primary endpoint of all-cause death. These patients died on average 5.5 months after BAV (range 0-15 months, median three months). Five patients (21.7%) reached the secondary endpoint: four deaths of cardiovascular or unknown cause, and one readmission due to heart failure during follow-up. Readmission due to stroke or myocardial infarction did not occur.
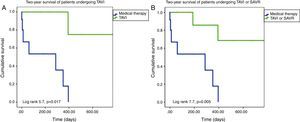
On Kaplan-Meier analysis, mortality was significantly lower among patients undergoing TAVI or SAVR at one-year follow-up (10.0% vs. 61.5%; p=0.005) and at two-year follow-up (20.0% vs. 84.6%; p=0.005) (Figure 2). Only definitive treatment was a predictor of survival (TAVI – hazard ratio [HR]: 8.5, 95% confidence interval [CI]: 1.1-69.3, p=0.05; TAVI or SAVR – HR: 7.4, 95% CI: 1.5-37.2, p=0.02), by Cox regression analysis. Age, left ventricular function, comorbidities (hypertension, dyslipidemia, diabetes, CAD, AF, CKD, malignancy, CPOD, liver disease), BAV indication, STS score and EuroScore II were not predictors of survival following BAV.
(A) Two-year Kaplan-Meier survival curves of patients undergoing TAVI after BAV vs. those maintained under medical therapy; (B) two-year Kaplan-Meier survival curves of patients undergoing TAVI or SAVR after BAV vs. those maintained under medical therapy. BAV: balloon aortic valvuloplasty; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation.
Although the number of procedures performed in our center was relatively low, this study represents the largest sample of patients undergoing BAV published in Portugal.
Patients with symptomatic severe AS and high surgical risk have seen an improvement in prognosis after the introduction and widespread use of TAVI. The TAVI procedure was introduced in Portugal in 2007 and in our center in 2012, and there is nationwide consensus regarding the use of this therapy.11 By January 2016, 155 valves had been implanted and 5.2% (n=8) of patients had BAV before TAVI.
As TAVI becomes more widely available, decisions regarding extremely high-risk patients will become a challenge. Similarly to other registries, this procedure was most frequently performed as a bridge to TAVI or as definitive therapy. A significant post-procedural reduction in aortic gradient was observed, with an acceptable rate of periprocedural complications and mortality, considering the high risk profile of this population, and in agreement with previously published high-volume series.12,13
Long-term outcome in BAV patients bridged to TAVI or SAVR is superior to outcome in palliative use alone.13,14 Moreover, patients who were successfully bridged to TAVI or SAVR had similar survival rates to those who had undergone primary TAVI or SAVR; however, without further interventions, the survival rate after BAV was similar to that of medical therapy alone.12,13,15 Our registry confirms the poor long-term prognosis for patients with symptomatic severe AS undergoing BAV alone, with mortality of up to 55.6% at six months. This observation is in agreement with previously reported rates. Liberman et al. found survival rates of 52%, 31%, and 18% at one, two, and three years after BAV, respectively.16 Otto et al. reported 55% survival at one year, 35% at two years, and 23% at three years.6 At one year, most patients died for non-cardiac reasons, due to the burden of comorbidities, and all-cause death was significantly higher in patients who did not undergo TAVI or SAVR. Of note, 26.1% of patients had concomitant active malignancy. Such patients are difficult to treat, because of the inability to pursue therapy for either malignancy or severe AS.
Subgroup analysis further corroborates the overall results, as patient outcome is ultimately determined by definitive treatment for severe AS, and not by the indication itself. BAV can help in recovering from a critical condition or enabling the patient to undergo non-cardiac surgery, so that TAVI or surgery can be performed in a more stable setting, aiming for a better outcome.
Predictors of mortality in patients undergoing BAV have been previously reported, including baseline functional status, cardiac output, renal function, cachexia, female gender, left ventricular systolic function, and mitral regurgitation.17,18 In the present registry, only the absence of definitive treatment predicted mortality, which may be explained by the small population size and the high mortality rate. Symptomatic severe AS was a major determinant of mortality by itself, diluting the potential impact on mortality of other variables.
Practical recommendationsIn light of our results and the limited availability of TAVI (since the prosthesis is not yet available off the shelf), we believe BAV should primarily be used as a bridge to definitive therapy (accepted or intended). This includes (1) patients who require non-cardiac procedures such as surgery or treatment for infection or cancer, (2) outpatients on a waiting list who are deteriorating clinically, and (3) inpatients with acute heart failure due to AS refractory to medical therapy (such as ventilated patients who cannot be weaned).
We believe BAV primarily for palliative purposes is generally not a good option, as these patients die shortly afterwards if no definitive treatment is undertaken, and essentially exposes patients to a procedure that is prone to complications but with no impact on prognosis. However, in some cases the patient's prognosis may not be absolutely clear. Thus, an individualized, multidisciplinary approach should be pursued.
Regarding timing, as our results and those of others clearly demonstrate, BAV provides an effective yet short-lived improvement, with a mean survival of 5.5 months and many patients dying within only three months. Thus, definitive treatment should ideally be offered as soon as possible and no later than three months after BAV, although we realize this may be difficult due to limited availability of prosthetic valves.
Study limitationsOur study has limitations, mainly related to its retrospective design, and to the assessment of complications according to the VARC-2 definitions, which have been described and updated specifically for TAVI patients. Echocardiographic follow-up was performed at the discretion of the attending clinicians rather than routinely, which may limit the value of comparisons of hemodynamic data from echocardiography.
ConclusionsIn the TAVI era, BAV is a safe and effective procedure and should be considered for selected patients with severe AS and temporary contraindications to definitive therapy or as palliative therapy. Given the limited availability of TAVI, our data suggest that BAV should more often be considered in patients with high risk and temporary contraindications or who are on a waiting list for TAVI, as it may lead to improvements in clinical condition and survival. However, long-term survival is poor after BAV alone, so TAVI or SAVR should be performed during follow-up. As TAVI develops and is performed more frequently, we expect that the use of BAV will increase, and will have an important role in the complex treatment algorithm for high-risk patients with severe AS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo specific funding grants were used regarding this study.
Conflicts of interestThe authors have no conflicts of interest to declare.