Coronary artery fistulas are the second most frequently seen coronary anomaly following abnormalities of coronary artery origin and distribution. A coronary fistula is defined as a direct communication between a coronary artery and any cardiac chamber or vessel. Treatment options include percutaneous embolization and surgical intervention. Herein, we present a case of a giant coronary artery fistula and right atrial tachycardia that was induced during a diagnostic electrophysiologic study but was not inducible after the successful treatment of the fistula. This is the first case indicating this association.
As fístulas coronárias são a segunda anomalia mais frequente das artérias coronárias a seguir às anomalias coronárias da câmara de saída. A fístula define-se como uma comunicação direta entre as artérias coronárias e uma cavidade cardíaca ou estrutura vascular. As opções terapêuticas incluem a embolização percutânea e o tratamento cirúrgico. Apresentamos aqui um caso de uma fistula coronária gigante e taquicardia auricular direita induzida durante um estudo electrofisiológico diagnóstico e que não foi possível induzir após o tratamento bem sucedido da fístula. Este é o primeiro caso que reporta esta associação.
Congenital coronary artery fistula (CAF) is defined as a direct communication between a coronary artery and any cardiac chamber or vessel.1 Although rare, they are the most common hemodynamically significant congenital coronary artery anomaly.1,2 Patients may be asymptomatic, but may also present with symptoms such as angina, exertional dyspnea, fatigue, or heart failure depending on the hemodynamic significance of the fistula.2 Transcatheter embolization has been an increasingly popular treatment following the first fistula occlusion by Reidy et al. in 1983.3 Following reports in the literature, we present a successful transcatheter occlusion of a giant fistula by coil embolization in a patient who presented to our outpatient clinic with complaints of exertional dyspnea, chest pain, fatigue, and paroxysmal palpitations.
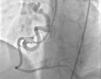
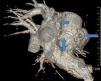
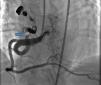
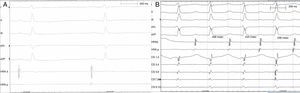
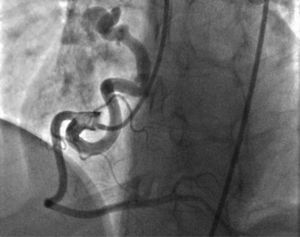
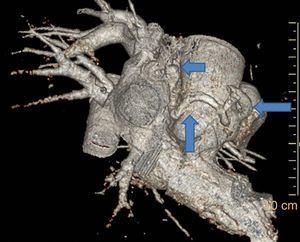
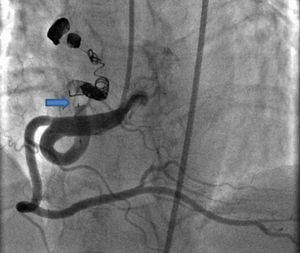
Case reportA 49-year-old woman was admitted to our outpatient clinic with complaints of exertional dyspnea, exertional chest pain, paroxysmal palpitation attacks, and fatigue. She had been treated for diabetes for two years but had no additional risk factors for coronary artery disease. On physical examination, her blood pressure was 130/80 mmHg and heart rate 85 bpm. No pathological sounds were heard during pulmonary and cardiac auscultation. Routine biochemical and hemogram values were within normal ranges. Ejection fraction was 60%, and no serious valve disease was detected on transthoracic echocardiography. Electrocardiography showed sinus rhythm. Right atrial tachycardia was induced with programmed atrial stimulation during a diagnostic electrophysiologic study (Figure 1). Coronary angiography was also performed in order to clarify potential ischemic etiology. While the left coronary and right coronary arteries were found to be normal, a giant fistula was detected from the proximal portion of the right coronary artery to the right atrium (Figure 2). Qp/Qs was 1:1 by oximetry and all right heart pressures were within normal ranges during right heart catheterization. Computed tomography (CT) angiography was also conducted for a detailed anatomical assessment, which showed a fistulized artery about 8 mm in diameter originating from the right coronary artery 2 cm distal to the orifice and draining into the intersection of the inferior vena cava and right atrium after following a tortuous course (Figure 3). The patient's symptoms were considered to be related to a coronary steal phenomenon in addition to the arteriovenous shunt caused by such a large fistula. A percutaneous occlusion procedure was preferred for the patient's comfort and to avoid complications likely to be caused by surgery. During the procedure, a Judkins guiding catheter was placed in the right coronary artery ostium and the fistulized artery was selectively catheterized via microcatheter, then multiple coils with different sizes ranging from 9 mm to 3 mm were sequentially placed in a suitable location in the mid portion of the fistulized artery until total occlusion was achieved (Figure 4). The patient's atrial tachycardia could not be induced again during the control electrophysiologic study repeated at the end of one month. Finally, progressive improvement was observed in exertional capacity, and other symptoms including paroxysmal palpitations completely disappeared during follow-up.
Electrocardiographic tracings during sinus rhythm (A) and atrial tachycardia (B). Variability of ventricular activity duration and the first atrial activity seen on proximal coronary sinus recordings indicate left atrial tachycardia (B). Recording speed 150 mm/s, tachycardia cycle length 420 ms.
A fistulized artery originating from the right coronary artery 2 cm distal to the orifice and draining into the inferior vena cava at the intersection with the proximal atrium after following a tortuous course, as demonstrated by reformatted three-dimensional volume rendered computed tomography angiographic imaging.
Congenital CAF is defined as the presence of a direct communication between a coronary artery and any cardiac chamber or vessel.1 Although rare, they are the most common hemodynamically significant congenital coronary artery anomaly.1,2 Fistulized arteries may be open to either the systemic or the pulmonary circulation. They may be congenital or acquired. The pathophysiological mechanism in congenital CAF is described as continuance of the sinusoidal connections which maintain myocardial blood flow in the early embryological period. Acquired CAF may be caused by cardiac trauma, surgery, percutaneous intervention or pacemaker installation, or be secondary to inflammation.3 In their series of 126595 cases of patients undergoing coronary angiography, Yamanaka and Hobbs reported an incidence of coronary artery anomalies of 1.3%, while that of CAF was 0.18% (13% of all coronary artery anomalies).4 Fistulized arteries, originating in the right coronary artery or its branches, comprise 50–55% of all CAFS. In terms of the structures with which coronary fistulas are connected, 40% are fistulized to the right ventricle, 25% to the right atrium, 15–20% to the pulmonary artery, and 7% to the coronary sinus.5 In our case, a giant fistula was detected originating in the proximal right coronary artery and draining into the intersection of the inferior vena cava and the right atrium.
CAFs do not give rise to symptoms or complications in most cases, and so most are detected either incidentally during coronary angiography or in the course of examinations to clarify a cardiac murmur.5 Small CAFs are generally asymptomatic and rarely expand, and may close spontaneously, especially those that open into the right ventricle. On the other hand, large fistulas tend to continue to expand, and despite not causing any symptoms in the first two decades of life, symptoms and complications may develop with age,1,6 including heart failure, myocardial ischemia and angina, infective endocarditis, and atrial fibrillation. Myocardial ischemia and angina may develop in large fistulas due to a coronary steal phenomenon.1 Angina has been reported in 7% of cases, myocardial infarction in 3%, and heart failure in 12–15%.6 Fistula-related mortality is correlated with surgical intervention, and has been reported as occurring in 0–4% of cases. The surgical mortality risk is higher in large aneurysmal fistulas and in those originating in the right coronary artery and draining into the left ventricle than in other cases.6 No association between CAFs and atrial tachycardia has so far been indicated in the literature.
Atrial tachycardia is a type of supraventricular tachycardia that originates from a focus in either of the two atria with a heart rate of >100 bpm. The symptoms of atrial tachycardia are similar to those of other supraventricular tachycardias: palpitations, lightheadedness, dizziness, shortness of breath, reduced exercise capacity, weakness, fatigue, chest discomfort, and sweating episodes. Atrial tachycardia is seen in patients both with and without structural heart disease. It can originate from virtually any focus in the left or right atrium. Therapy for patients suffering from atrial tachycardia depends on the frequency and severity of symptoms and includes either medical management or curative catheter ablation.
CAF can be diagnosed by means of the physical examination findings in asymptomatic patients. The point where the continuous murmur related to the CAF is loudest may vary depending on the fistula's entry site into the heart. While those that end in the right atrium cause a murmur at the lower sternal border, in those ending in the pulmonary artery a murmur is heard at the second intercostal space, and in those ending in the left ventricle the murmur may be near the apex.7 In the case presented, no pathological sounds were heard during cardiac auscultation. However, the nature of the patient's paroxysmal palpitations was characteristic of a supraventricular tachycardia, so we immediately decided to perform a diagnostic electrophysiologic study. Additionally, the patient's history of diabetes, exertional angina, and other symptoms of heart failure prompted suspicions in this case concerning an ischemic etiology, which prompted us to perform coronary angiography as well. Although this is the gold standard for imaging the coronary arteries, it may be insufficient to assess the fistula's relation with nearby anatomical structures. CT provides detailed information on the origin and drainage sites.3 For this reason, our patient also underwent CT angiography after the fistula was diagnosed by coronary angiography. Right heart catheterization was also performed to assess the fistula's hemodynamic significance; the normal right heart catheterization findings were considered to be due to the fact the fistula drained into the intersection of the inferior vena cava and right atrium.
Unless small and asymptomatic, CAFs should be corrected due to the increased risk of thrombosis, endocarditis, rupture, and heart failure. Asymptomatic cases require close follow-up by echocardiography or angiography.6,7 Treatment options include transcatheter occlusion and surgery. Described for the first time by Reidy et al. in 1983, transcatheter occlusion is increasingly used,3 although tortuous and extra-large fistulas with more than one opening and with serious aneurysmal dilatations are not suitable for a transcatheter approach.8 Transcatheter coil implantation is the method of choice in suitable cases. Complications of the transcatheter approach are related to the catheter and guidewire manipulation, as well as coil placement in an unsuitable location or embolization.8 However, the transcatheter approach is superior to surgery in that it increases patient comfort and avoids surgical complications.6 However, in cases unsuitable for a transcatheter approach, surgical treatment is an option, involving median sternotomy and cardiopulmonary bypass.2,8 Risk of post-surgical myocardial infarction is reported as 3%, mortality 2–2.4%, and fistula recurrence 4%.1 With increasing experience and technical advances, transcatheter occlusion has become a successful method. Its reliability and effectiveness are equal to those of surgery, and it is thus preferable in most cases with a hemodynamically important fistula.6,8
In our patient, it was decided to intervene due to the presence of severe symptoms associated with coronary steal and high-output heart failure, and we opted for transcatheter coil embolization after assessing the suitability of the fistulized artery's anatomical features for the procedure. The fistula was successfully occluded by transcatheter coil embolization, with no complications. Although right atrial tachycardia, to which most of the patient's symptoms can be attributed, was induced during an electrophysiologic study, we suspected concomitant coronary artery disease. We therefore performed coronary angiography before deciding on any treatment for atrial tachycardia. After detecting a giant right coronary fistula and excluding coronary artery disease, we hypothesized that the patient's atrial tachycardia might have been caused by this fistula, so we opted to treat it first and treat the atrial tachycardia later. The tachycardia could not be induced again during the control electrophysiologic study repeated after one month; the patient stated that her symptoms, including paroxysmal palpitations, had completely disappeared, and her exertional capacity was also substantially improved during the follow-up period.
All CAFs that cause hemodynamic compromise or serious symptoms require intervention. Although CAF is rarely seen, it should be borne in mind that atrial tachycardia may be associated with CAFs, especially those draining into the atria. Therefore, vascular disorders such as CAF should be considered before specifically treating these arrhythmias.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.