Arterial hypertension is a major risk factor for cardiovascular and renal events. Lowering blood pressure is thus an important strategy for reducing morbidity and mortality. Since low-dose aspirin is a cornerstone in the prevention of adverse cardiovascular outcomes, combined treatment with aspirin and antihypertensive drugs is very common. However, the impact of aspirin therapy on blood pressure control remains a subject of intense debate.
Recent data suggest that the cardioprotective action of aspirin extends beyond its well-known antithrombotic effect. Aspirin has been shown to trigger the synthesis of specialized pro-resolving lipid mediators from arachidonic acid and omega-3 fatty acids. These novel anti-inflammatory and pro-resolving mediators actively stimulate the resolution of inflammation and tissue regeneration. Additionally, they may contribute to other protective effects on redox status and vascular reactivity that have also been attributed to aspirin. Of note, aspirin has been shown to improve vasodilation through cyclooxygenase-independent mechanisms. On the other hand, higher aspirin doses have been reported to exert a negative impact on blood pressure due to inhibition of cyclooxygenase-2 activity, which reduces renal blood flow, glomerular filtration rate and sodium and water excretion.
This review aims to provide an overview of the effects of aspirin on blood pressure and the underlying mechanisms, focusing on the interaction between aspirin and antihypertensive drugs. Studies in both experimental and human hypertension are presented.
A hipertensão arterial representa um fator de risco major para eventos cardiovasculares e renais. Por esse motivo, a redução da pressão arterial é uma estratégia importante para a diminuição da morbilidade e mortalidade. Como a aspirina em dose baixa constitui a terapêutica base na prevenção de eventos cardiovasculares, a sua associação com fármacos anti-hipertensores é muito comum. No entanto, o impacto da aspirina no controlo da pressão arterial permanece um tema de intensa discussão.
Estudos recentes sugerem que a ação cardioprotetora da aspirina não está limitada ao seu conhecido efeito antitrombótico. A aspirina ativa a síntese de mediadores pró-resolutivos especializados a partir do ácido araquidónico e de ácidos gordos ómega-3. Estes novos mediadores anti-inflamatórios e pró-resolutivos estimulam ativamente a resolução da inflamação e a regeneração tecidual. Adicionalmente, poderão contribuir para os efeitos protetores no estado redox e na reatividade vascular que têm sido atribuídos à aspirina. É de sublinhar que a aspirina parece também melhorar a vasodilatação por mecanismos independentes da inibição da cicloxigenase. Por outro lado, o uso de aspirina em doses altas parece exercer um efeito negativo na pressão arterial devido à inibição da atividade da cicloxigenase-2 e consequente redução do fluxo sanguíneo renal, da taxa de filtração glomerular e da excreção de sódio e água.
Este artigo pretende rever os efeitos da aspirina na pressão arterial e mecanismos subjacentes, com enfoque na interação entre a aspirina e os fármacos anti-hipertensores. São apresentados estudos na hipertensão experimental e humana.
15R-epimeric lipoxin A4
15R-epimeric lipoxin B4
15-epi-lipoxins
15(R)-hydroxyeicosatetraenoic acid
17R-hydroxydocosahexaenoic acid
18R-hydroxyeicosapentaenoic acid
5-lipoxygenase
arachidonic acid
ambulatory blood pressure measurement
angiotensin-converting enzyme inhibitors
angiotensin II
angiotensin receptor blockers
aspirin triggered protectin
aspirin triggered resolvin D1
aspirin triggered resolvin D2
aspirin triggered resolvin D3
aspirin triggered resolvin D4
aspirin-triggered lipoxins
beta-blockers
blood pressure
cyclic guanosine monophosphate
cyclooxygenase-1
cyclooxygenase-2
diastolic blood pressure
docosahexaenoic acid
endothelial nitric oxide synthase
eicosapentaenoic acid
hygienic-dietary recommendations
Hypertension Optimal Treatment
Losartan Intervention For Endpoint reduction in hypertension
lipoxygenase
lipoxins
nicotinamide adenine dinucleotide phosphate oxidase
nuclear factor kappa B
nitric oxide
nonsteroidal anti-inflammatory drugs
superoxide
protectins
prostaglandin E2
prostacyclin
prostaglandins
plasma renin activity
proline-rich tyrosine kinase 2
reactive oxygen species
resolvin E1
resolvin E2
resolvins
systolic blood pressure
spontaneously hypertensive rats
Sprague Dawley
smooth muscle cells
specialized pro-resolving lipid mediators
thromboxane A2
thromboxane B2
Wistar-Kyoto rats
Arterial hypertension affects 30-45% of the general population in Europe.1 In Portugal, its prevalence was found to be 42.2%, with a rate of blood pressure (BP) control of only 55.7% among treated patients.2 Hypertension is a major risk factor for cardiovascular and renal events1,3 and lowering BP is an important strategy for reducing morbidity and mortality.3,4
Associating antihypertensive medication with correction of other cardiovascular risk factors is also an important way to increase treatment benefits in hypertensive patients. Low-dose aspirin plays an essential role in the prevention of adverse cardiovascular outcomes, especially in patients with previous cardiovascular events.1,5–7 Although it is commonly combined with antihypertensive medication, aspirin therapy is controversial in individuals without a history of cardiovascular disease. According to the 2013 European guidelines for the management of hypertension, low-dose aspirin should be prescribed for controlled hypertensive patients with previous cardiovascular events and considered for hypertensive patients with renal dysfunction or high cardiovascular risk, but is not recommended for low-to-moderate risk hypertensive patients in whom absolute benefit and harm are equivalent.1 Similar recommendations on aspirin use have been reported in the recent European guidelines for cardiovascular disease prevention.8
The impact on BP of the combination of aspirin with first-line antihypertensive drugs, such as beta-blockers (BBs), angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), has also been the subject of intense debate. There are reports of negative interactions with BBs or ACEIs,9–12 while other studies have shown no influence on the BP-lowering effect of ACEIs and ARBs.13–15 Discussion of the benefits and harms of aspirin therapy in patients taking antihypertensive drugs extends to its effects on other cardiovascular parameters or endpoints.15–17
The present review aims to provide an overview of the effects of aspirin on blood pressure and the underlying mechanisms, focusing on the interaction between aspirin and antihypertensive drugs.
Aspirin and inhibition of cyclooxygenase-1 and cyclooxygenase-2Aspirin's pharmacological effects have been attributed to the inhibition of cyclooxygenase (COX) enzymatic activity and further arachidonic acid (AA) metabolism.18 COX-1 is a constitutively expressed enzyme isoform responsible for the synthesis of prostanoids from AA. These include thromboxane A2 (TXA2) and prostaglandins (PGs), which mediate various homeostatic functions such as gastric protection, renal blood flow regulation and platelet aggregation. In contrast, the COX-2 isoform is mostly expressed in response to inflammatory stimuli, mitogens and growth factors, and generates PGs involved in pain signaling, fever and tissue repair, although it is also constitutively expressed in the macula densa and renal medulla, contributing to the regulation of kidney function.18–21
The irreversible acetylation of COX-1 by low-dose aspirin reduces TXA2 biosynthesis in platelets, which inhibits platelet aggregation, thus contributing to cardioprotection.18,22 In addition, the decrease in TXA2 levels may also account for the attenuation of vasoconstriction.18,23 Higher aspirin doses are required to block COX-2 activity and achieve analgesic, antipyretic and anti-inflammatory effects. COX-2 blockade decreases the synthesis of prostacyclin (PGI2), a potent vasodilator and inhibitor of platelet aggregation, and of other PGs such as prostaglandin E2 (PGE2) that are involved in the control of BP and renal function.18,21,22,24
Aspirin and production of pro-resolving lipid mediatorsIn recent years, a new paradigm of the inflammatory process has emerged, with the discovery of specialized pro-resolving lipid mediators (SPMs) such as lipoxins (LXs), resolvins (Rvs) and protectins (PDs), that actively stimulate the resolution of inflammation and tissue regeneration.25 SPMs are biosynthesized in transcellular processes involving AA or omega-3 fatty acids as precursors and the cooperation of several lipoxygenases (LOXs).26
Interestingly, the acetylation of COX-2 by aspirin triggers the production of SPMs. In contrast to what occurs with COX-1, which becomes irreversibly inhibited, COX-2 acetylation by aspirin only changes its activity profile, yielding 15(R)-hydroxyeicosatetraenoic acid (15R-HETE) from AA, instead of the PG precursor PGH2. 15R-HETE is further metabolized into 15R-epimeric LXA4 (15-epi-LXA4) or 15R-epimeric LXB4 (15-epi-LXB4), which are also known as aspirin-triggered LXs (ATL) (Figure 1).26–28 Of note, this effect appears to be aspirin-specific, since it is not shared by other nonsteroidal anti-inflammatory drugs (NSAIDs).27,29 LXs and ATL exert several anti-inflammatory and pro-resolving effects, including inhibition of neutrophil recruitment, stimulation of neutrophil apoptosis and removal by macrophages, inhibition of proinflammatory cytokine synthesis, and stimulation of anti-inflammatory cytokine production.26 LXs and ATL also inhibit generation of reactive oxygen species (ROS), induce expression of antioxidant molecules and stimulate nitric oxide (NO) and PGI2 production and endothelium-dependent vasodilation.26,30 ATL biosynthesis has been demonstrated in healthy subjects treated with low-dose aspirin for eight weeks. Since ATL was generated in sufficient levels to achieve anti-inflammatory and pro-resolving effects, it is conceivable that it contributes to the cardiovascular benefits of aspirin.29 Our group also reported that patients with mild-to-moderate chronic heart failure treated with low-dose aspirin had a four-fold higher urinary excretion of ATL compared to untreated patients.31 Of note, it has recently been shown that LXs and ATL promote reverse cholesterol transport, thus suggesting that these SPMs may be promising therapeutic allies in cardiovascular disease prevention.32,33
Aspirin-acetylated COX-2 also triggers the production of other SPMs from omega-3 fatty acids. Aspirin-triggered D-series Rvs and aspirin-triggered PDs are generated from docosahexaenoic acid, while E-series Rvs are produced from eicosapentaenoic acid (Figure 2). These SPMs also exert anti-inflammatory and pro-resolving effects, as well as protective effects on redox status and vascular reactivity.26
Biosynthesis of aspirin-triggered resolvins and protectin. 5-LOX: 5-lipoxygenase; 17R-HDHA: 17R-hydroxydocosahexaenoic acid; 18R-HETE: 18R-hydroxyeicosapentaenoic acid; AT-RvD1: aspirin-triggered resolvin D1; AT-RvD2: aspirin-triggered resolvin D2; AT-RvD3: aspirin-triggered resolvin D3; AT-RvD4: aspirin-triggered resolvin D4; AT-PD: aspirin-triggered protectin D; COX-2: cyclooxygenase-2; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; RvE1: resolvin E1; RvE2: resolvin E2.
Oxidative stress contributes to the pathogenesis of hypertension.34–39 Given the interplay between inflammation and oxidative stress,40 it has been suggested that aspirin might have antioxidant properties that account for its cardiovascular benefits. Wu et al. observed that treatment with aspirin for 12 days significantly dose-dependently (10-100 mg/kg/day) reduced vascular superoxide (O2−) production by lowering nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in normotensive Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR). Aspirin also restored acetylcholine-induced vasorelaxation in aortic rings from SHR, but not from WKY. In Sprague Dawley (SD) rats, aspirin also inhibited angiotensin II (Ang II)-induced O2− generation and attenuated the progression of hypertension. Furthermore, long-term treatment initiated in normotensive young SHR attenuated the development of hypertension, but did not alter BP in young WKY or in adult SHR.41 Wu et al. further demonstrated that treatment of SD rats with aspirin (100 mg/kg/day for 12 days) prevented Ang II-induced production of O2− in cardiovascular tissues, as well as O2− and protein synthesis in cultured smooth muscle cells (SMC). It also prevented Ang II-induced hypertension and cardiac hypertrophy. In contrast, other NSAIDs failed to prevent Ang II-induced effects in vivo.42
In cultured mice cardiac fibroblasts, pretreatment with aspirin or salicylic acid in therapeutically relevant concentrations suppressed Ang II-induced NADPH oxidase expression, ROS generation and activation of the transcription factor nuclear factor-kappa B (NF-κB).43
Of note, ATL also exerts protective effects on redox status. In endothelial cells, treatment with ATL blocked NADPH oxidase-mediated ROS production and increased the expression of the antioxidant molecule heme-oxygenase-1.44,45 ATL also inhibited ROS generation in leukocytes.46,47
Aspirin and vascular toneVascular tone is a determinant of peripheral resistance and consequently of BP. Aspirin and salicylates may lower BP through a COX-independent mechanism involving the inhibition of a non-receptor tyrosine kinase, proline-rich tyrosine kinase 2 (PYK2), in vascular SMCs.48,49 PYK2 is activated by various physiological stimuli including Ang II, and seems to be essential for RhoA/Rho kinase activation, which is critical for calcium sensitization of vascular smooth muscle and appears to be increased in hypertension.48,50 Both aspirin and sodium salicylate relaxed preconstricted vessels by inhibiting PYK2-mediated RhoA/Rho-kinase activation. This occurred at higher concentrations than those necessary to inhibit COX.48
Exploratory clinical trials have also suggested that low-dose aspirin improves endothelium-dependent vasodilation51–53 and it has therefore been hypothesized that aspirin might stimulate the release of NO from the vascular endothelium.54 Taubert et al. demonstrated that low concentrations of aspirin elicited the release of NO from vascular endothelium and increased the activity of endothelial NO synthase (eNOS). This effect appears to be independent of COX but related to direct acetylation of eNOS, and seems to be selective for aspirin since salicylate and indomethacin failed to modulate NO release. Additionally, higher aspirin concentrations scavenged O2−, thus preventing NO degradation.54
The major protective effects of aspirin at low and medium/high doses are summarized in Figures 3 and 4.
The BP effects of aspirin were studied in two models of renovascular hypertension: in one kidney, one clip (1K1C)-hypertensive rats, and in two kidney, one clip (2K1C)-hypertensive rats. Male SD rats were treated twice a day with aspirin 100 mg/kg or vehicle and, four days after beginning treatment, their left renal artery was clipped (2K1C rat model). In another group, besides left renal artery clipping, the right kidney was also removed in all animals (1K1C rat model). BP was measured three weeks after surgery. In comparison with vehicle-treated 2K1C rats, aspirin-treated 2K1C rats exhibited higher BP values (160 mmHg vs. 140 mmHg, p<0.05). Interestingly, aspirin treatment attenuated the BP increase in 1K1C rats (153 mmHg vs. 208 mmHg, p<0.05). Since 1K1C and 2K1C rats displayed different profiles in response to AA administration, with 1K1C rats exhibiting an enhanced vasodepressor response to AA, the authors suggested that aspirin decreases BP in hypertensive rats with increased activity of the COX pathway and raises BP in hypertensive rats that have a ‘normal’ COX pathway.55
Tuttle et al. also assessed the antihypertensive effect of aspirin in SHR and WKY. They first demonstrated that prolonged administration of aspirin (100 mg/kg/daily) to young SHR prevents the development of hypertension.56 Since aspirin loses its antihypertensive effect in adult SHR, they investigated whether the loss of aspirin's effect on BP was associated with aging or with the duration of exposure to aspirin treatment. A loss of aspirin's antihypertensive effect was found in all treated groups when SHR and WKY reached 110 days of age. Furthermore, since changes in PGF2α, used as an index of COX activity, did not parallel the BP alterations, the authors assumed that the age of the animal, and not a change in enzymatic activity or process over time, was responsible for the loss of aspirin's antihypertensive effect.56 Similar results were obtained by Wu et al., who observed significant attenuation of the development of hypertension in young SHR treated with aspirin (100 mg/kg/day for 53 days) but not in older aspirin-treated SHR or WKY.41 Nevertheless, aspirin treatment significantly reduced aortic O2− production in both old SHR and WKY.41
The effect of aspirin on BP in SHR also appears to depend on baseline systolic BP (SBP) values. In four-to-six-month old SHR, a three-day treatment with aspirin (100 mg/kg/day) reduced SBP in the SHR group exhibiting SBP values above 161 mmHg before treatment. In contrast, in other SHR with baseline SBP below 160 mmHg, aspirin significantly increased SBP, and this elevation was more marked in SHR that had baseline normotensive SBP values. Although the authors found no differences in the aortic production of TXA2 and PGI2, they speculated that the BP effects of aspirin were caused by inhibiting production of vasoconstrictor or vasodilator prostanoids.57
In Ang II-infused rats, concurrent aspirin treatment (100 mg/kg/day for 12-14 days) completely prevented Ang II-induced production of O2− in cardiovascular tissues, BP rise and cardiac hypertrophy. These effects were not shared by other NSAIDs.42 When aspirin was given to Ang II-infused rats after the establishment of hypertension, BP was significantly reduced after three days of treatment.42 In chronically glucose-fed rats with elevated BP, treatment with aspirin (100 mg/kg/day for three weeks) also prevented rises in SBP, glucose levels and aortic O2− production, and attenuated insulin resistance.58
The aspirin dose (100 mg/kg) used in these studies is high, being equivalent to a 1-g dose for a 70-kg man. Nevertheless, a lower dose (10 mg/kg/day), which would be equivalent to 100 mg/day in a man, was shown to significantly reduce vascular O2− production.42 These antioxidative properties may account for the cardiovascular benefits of low-dose aspirin.
The impact of lower aspirin doses on BP has been tested in some studies. In young male SHR, the administration of aspirin (10 mg/kg) twice a week for 10 weeks had no effect on SBP.59,60 In male Wistar rats, 50 mg/kg aspirin elicited a pressor response that was markedly enhanced by pretreatment with celecoxib, a COX-2 inhibitor, or with zileuton, a 5-LOX inhibitor, or with Boc-2, a LXA4 receptor antagonist.30 Furthermore, aspirin increased the serum concentration of ATL 20-fold and the administration of LXA4 alone significantly decreased BP. Thus, the formation of ATL attenuates the pressor response to aspirin, and drugs that inhibit ATL production enhance the aspirin-induced BP rise.30 In another study, treatment with aspirin (53.5 mg/kg/day) for seven days did not alter BP in male Wistar rats but significantly reduced BP in stroke-prone SHR compared to vehicle-treated animals.61 Our group recently assessed the effect of low-dose aspirin (10 mg/kg/day for eight days) alone or in combination with losartan in male WKY and SHR. Regarding the effect of aspirin alone, no significant changes were observed in SBP of WKY or SHR compared to their respective controls.62 Recently, another study also confirmed the lack of effect of low-dose aspirin (10 mg/kg/day for seven days) on the BP of normotensive WKY.63
Effects when used in combination with other antihypertensive drugsThe impact on BP of the combination of aspirin with antihypertensive drugs has been little studied. Tuttle et al. studied the antihypertensive efficacy of the BB metoprolol, aspirin (75-100 mg/kg/day), and their combination for 56 days, in young SHR and WKY. Treatment with metoprolol or aspirin alone prevented BP rise in both WKY and SHR. In contrast, when the drugs were co-administered, a loss of the antihypertensive effect was observed in SHR but not in WKY. Renal PGs were also measured after 56 days of treatment. Whereas renal PG content was higher in older SHR than in age-matched WKY, lower levels were found in SHR receiving aspirin or metoprolol. SHR treated with both drugs also exhibited reduced renal PG content, despite the loss of antihypertensive efficacy. The authors concluded that aspirin and metoprolol attenuate each other's antihypertensive effect, but could not explain the underlying mechanism based on the changes observed in renal PGs.11
Our group assessed the effects of combined treatment with low-dose aspirin (10 mg/kg/day for eight days) and the ARB losartan in male SHR and WKY. Although aspirin treatment did not modify the antihypertensive effect of losartan in WKY, it significantly improved the BP-lowering efficacy of losartan in SHR. This effect was associated with a significant reduction in renal COX-2 expression and in the production of vasoconstrictive eicosanoids, compared to SHR treated with losartan alone.62,64
The interaction between aspirin and ACEIs was studied in SHR and in rats with methylprednisolone-induced hypertension. In male SHR, treatment with aspirin (200mg/kg/day for seven days) did not alter the antihypertensive effect of captopril, although it markedly inhibited the formation of vasodilator prostanoids. Thus, these prostanoids do not appear to contribute to the antihypertensive effect of captopril in SHR.65 However, in another study in older SHR, combined treatment with captopril and aspirin (200 mg/kg/day) for seven days enhanced the antihypertensive effect of captopril, apparently by inducing an increase in diuresis. The inhibition of renal PG excretion by aspirin was a transient effect and it was concluded that the enhancement of captopril's antihypertensive efficacy by aspirin was independent of renal PG excretion.66 In male Wistar rats with methylprednisolone-induced hypertension, treatment with 25 mg/kg/day or 100 mg/kg/day aspirin did not modify the hypotensive effect of lisinopril. However, cardiac and renal degenerative changes were found even with the lower aspirin dose, and the combination of the higher aspirin dose and lisinopril was associated with a significant increase in mortality compared to hypertensive rats treated with lisinopril alone.67 A summary of these studies is presented in Table 1.
Interaction of aspirin with antihypertensive drugs in experimental models of hypertension.
| Study | Total no. of rats | No. of rats taking aspirin | Age | Aspirin dose | Duration of aspirin treatment | Antihypertensive treatment/dose | Effect of combined aspirin treatment | Other effects |
|---|---|---|---|---|---|---|---|---|
| DiNicolantonio et al.65 | 32 male SHR | 16 (8 with aspirin; 8 with aspirin+captopril) | 11-15 weeks | 200 mg/kg/day | 7 days | Captopril 30 mg/kg/day | No significant change in antihypertensive effect | ↓ production of vasodilator prostanoids from exogenous AA with aspirin |
| Tuttle et al.11 | 31 male SHR 31 male WKY | 25 SHR 25 WKY | 28 days | 75-100 mg/kg/day | 56 days | Metoprolol 0.75-1.0 mg/kg/day | Loss of the antihypertensive effect in SHR but not in WKY | Metoprolol or aspirin alone prevented BP rise in WKY and SHR ↑ renal PG content (PGF1α+PGF2α) in older SHR (vs. age-matched WKY) ↓ renal PG content was found in SHR receiving aspirin, metoprolol or both |
| Quilley et al.66 | 40 male SHR | 8-10 rats with aspirin; 8-10 rats with aspirin+captopril | 20-40 weeks | 200 mg/kg/day | 7 days | Captopril 30 mg/kg twice/day | Aspirin ↑ the antihypertensive effect of captopril | Aspirin ↑ urine flow in SHR treated with captopril+aspirin (vs. captopril alone) ↓ in renal PG excretion only in the first 4 hours of aspirin administration |
| Dubey et al.67 | 24 male Wistar normotensive rats 32 male Wistar hypertensive rats induced by methylprednisolone (20 mg/kg per week, for 2 weeks) | 24 | Adults | 25 mg/kg/day 100 mg/kg/day | 2 weeks | Lisinopril 15 mg/kg/day | No significant change in the antihypertensive effect of lisinopril | Absence of structural abnormalities in cardiac and renal tissues of normotensive rats treated with lisinopril+aspirin or lisinopril alone Degenerative changes in hypertensive rats treated with aspirin+lisinopril (observed even with lower aspirin dose) Significant ↑ in mortality in hypertensive rats treated with lisinopril+high-dose aspirin (vs. lisinopril alone) |
| Sousa et al.62,64 | 32 male SHR 32 male WKY | 16 SHR (8 with aspirin; 8 with aspirin+losartan) 16 WKY (8 with aspirin; 8 with aspirin+losartan) | 12 weeks | 10 mg/kg/day | 8 days | Losartan 15 mg/kg/day | No significant change in the antihypertensive effect of losartan in WKY rats In SHR, ↑ in antihypertensive effect of losartan with aspirin (SBP:SHR+aspirin+losartan: 174.5±0.9 mmHg; SHR+losartan: 186.3±1.4 mmHg, p<0.05) | Significant ↓ of renal COX-2 expression and vasoconstrictive eicosanoids in SHR+aspirin+losartan (vs. SHR+losartan) |
↑: increase[d]; ↓: decrease[d]; AA: arachidonic acid; BP: blood pressure; COX-2: cyclooxygenase-2; PG: prostaglandins; SHR: spontaneously hypertensive rats; WKY: Wistar-Kyoto rats.
The influence of aspirin on BP in healthy subjects was investigated in a study designed to assess the effects of captopril, aspirin and the combination of both drugs on BP and on the excretion of prostanoids, nitrates and cyclic guanosine monophosphate (cGMP). A crossover trial was performed in 13 healthy females aged 24.9±0.5 years who were randomized to receive captopril (25 mg twice a day), aspirin (100 mg/day) or both drugs for one week, separated by a two-week washout. Aspirin did not change BP when given alone and did not modulate the hypotensive effect of captopril when co-administered. Urinary excretion of PGI2 metabolite, PGE2, nitrate and cGMP was not altered by either treatment but aspirin significantly reduced the excretion of TXA2 metabolite, when given alone or in combination with captopril.68
Hermida et al. investigated the administration time- and dose-dependent effects of aspirin on BP in healthy individuals. In their first study, 55 healthy subjects aged 20.9±1.8 years were randomized to receive aspirin (500 mg/day) for one week on awakening (n=18), during the afternoon (n=26) or at bedtime (n=11). There was a significant reduction in BP only when aspirin was administered during the afternoon.69 The same authors also conducted a double-blind, randomized clinical trial with 73 healthy participants (21.3±0.3 years of age) assigned to one of six groups defined according to placebo/aspirin dose (100 or 500 mg/day) for one week and to the circadian time of administration (awakening, afternoon and bedtime). In subjects receiving 500 mg aspirin, there was a small (approximately 2 mmHg) but significant reduction in SBP and diastolic blood pressure (DBP) when aspirin was given in the afternoon. Treatment with the lower dose in the afternoon elicited a significant SBP and DBP reduction, comparable to that obtained with 500 mg aspirin administered at the same time. However, the BP reduction was larger when 100 mg aspirin was given at bedtime. The higher impact on BP for 100 mg aspirin compared to 500 mg may be related to the selective inhibition of TXA2 but not PGI2 with the lower dose. The time dependency of BP reduction by aspirin could be due to circadian rhythms in circulating platelets, platelet aggregation, clotting and fibrinolytic inhibitors, and also in aspirin's inhibition of platelet aggregation.70
Aspirin and blood pressure in untreated hypertensive patientsHermida et al. also assessed the administration time-dependency of the effect of 100 mg/day aspirin for one week on BP reduction in untreated hypertensive subjects. This was performed in 18 volunteers aged 21.8±0.4 years, recently diagnosed with mild hypertension and untreated for hypertension. Aspirin significantly decreased BP when administered in the afternoon and at bedtime, but the effect was larger for bedtime administration.70 The same authors also conducted a trial in 100 untreated volunteers with mild (grade 1) essential hypertension, aged 42.5±11.6 years, who were randomly allocated to three groups to take hygienic-dietary recommendations (HDR) (n=50), HDR plus aspirin (100 mg/day) on awakening (n=24) or HDR plus aspirin (100 mg/day) before bedtime (n=26) for three months. Essentially, in the group that received aspirin before bedtime, but not in the other groups, there were significant reductions in SBP and DBP measured by conventional methods and by ambulatory blood pressure measurement (ABPM). Although the mechanisms responsible for this decrease have not been elucidated, the authors speculate that circadian rhythms in TXA2 production and in plasma renin activity (PRA) could account for the BP lowering effect of aspirin.71 Hermida et al. also conducted a similar study involving a larger sample of 328 untreated grade 1 hypertensive patients aged 44.0±12.6 years. These subjects were randomly distributed to the same groups described above (HDR, n=169; HDR plus aspirin 100 mg/day on awakening, n=77; HDR plus aspirin 100 mg/day before bedtime, n=82). The results obtained corroborated the previously described reduction of SBP and DBP values on ABPM and conventional measurement in the group receiving low-dose aspirin at bedtime.72 They further compared the chronobiological impact of aspirin on BP in dipper and non-dipper subjects with untreated grade 1 hypertension (n=257) randomized to receive aspirin (100 mg/day) on awakening (n=126, 80 dipper and 46 non-dipper) or at bedtime (n=131, 83 dipper and 48 non-dipper) for three months. This study again confirmed that aspirin given at bedtime, but not on awakening, significantly reduced 24-hour SBP and DBP. Of note, aspirin administered at bedtime induced a two-fold higher decrease in nocturnal BP in non-dipper patients compared to dipper patients.73 Interestingly, in another study they also demonstrated the existence of gender differences in BP response to aspirin administration. This trial included 130 men and 186 women with untreated grade 1 hypertension who were randomly assigned to take aspirin 100 mg/day on awakening or at bedtime for three months.74 When aspirin was administered on awakening, BP was not altered in men but was slightly increased in women. In contrast, when aspirin was given at bedtime, BP values were significantly decreased in both men and women. This reduction was significantly larger in women than in men, and gender differences in BP were greater during night-time rest.74
Magagna et al. also assessed the influence of 100 mg/day aspirin on BP control in untreated borderline or mild essential hypertensive subjects. Ten patients aged 47.9±5.1 years received aspirin (100 mg/day) or placebo for four weeks. Aspirin treatment did not modify BP in these patients but significantly reduced serum and urinary TXB2 and PRA.75 Although urinary excretion of PGI2 metabolite was not significantly altered by aspirin treatment, the authors reported that it was slightly decreased and could have contributed to the reduction in PRA.75 The effect on BP of low-dose aspirin in untreated hypertensive patients was further studied by Snoep et al. as a secondary endpoint in a crossover trial designed to assess the mechanisms underlying BP reduction when aspirin was given at bedtime.76 This trial included 16 untreated grade 1 hypertensive individuals aged 58.4±6.8 years who were randomly allocated to one of two groups, one receiving aspirin (100 mg/day) at bedtime and placebo on awakening, and the other receiving aspirin on awakening and placebo at bedtime, for two weeks. After a four-week washout interval, patients again received aspirin and placebo for another two weeks, in the reverse order of the first treatment period. The results of this study indicate that aspirin intake at bedtime compared with the morning significantly reduced PRA over 24 hours, as well as 24-hour urinary excretion of cortisol, dopamine and noradrenaline. The authors suggested that reduced activity of these pressor systems was a plausible explanation for the BP decrease when aspirin is given at night and the lack of effect detected when given in the morning. Although in this study no significant reduction in 24-hour BP was observed when aspirin was given at bedtime, the authors hypothesized that the treatment period was probably too short to translate the effects on BP regulating systems into effective BP reduction. They also underlined that their study was not designed and powered to detect differences in BP response to timed administration of aspirin.76
Aspirin and blood pressure in subjects at elevated risk for developing hypertensionIn addition to these studies in subjects with mild hypertension, Hermida et al. also compared the BP effects of different administration times (awakening, afternoon or bedtime) of low-dose aspirin (100 mg/day) in pregnant women at higher risk for gestational hypertension or preeclampsia.77–79 The results obtained in these trials corroborate previous findings in healthy and untreated mild hypertensive subjects,70 i.e., BP was significantly reduced when aspirin was administered in the afternoon and this effect was even greater when it was given at bedtime. There was no significant change in BP when aspirin was administered on awakening.77–79
Similar results were obtained in another study by the same group involving 244 individuals aged 43.0±13.0 years with prehypertension randomly distributed to one of three groups (non-pharmacological recommendations; the same recommendations and aspirin 100 mg/day on awakening; and the same recommendations and aspirin 100 mg/day at bedtime). Once again, a significant BP reduction was observed only when aspirin was given at bedtime.80
Aspirin and blood pressure in treated hypertensive patientsThe majority of small, short-term trials have reported that low doses (75-100 mg/day) of aspirin do not interfere with the BP lowering effect of ACEIs (captopril, enalapril), ARBs (losartan), BBs (atenolol) and calcium antagonists (nifedipine).10,14,19,75,81,82 Regarding the interaction of low-dose aspirin and ACEIs, although no change in the antihypertensive effect of ACEIs was detected, significant reductions in serum and/or urinary TXB2 were observed with combined treatment with aspirin compared with treatment with ACEIs alone.14,75,81 Nevertheless, no significant changes were observed in the urinary metabolite of PGI2.75 Studies assessing the effects of higher aspirin doses on the BP of patients treated with ACEIs have reported contradictory results. While Guazzi et al. showed that aspirin (300 mg/day) induced an antagonistic effect on the action of enalapril,10 Nawarskas et al. observed no significant changes in mean arterial pressure, SBP or DBP in patients treated with aspirin (325 mg) and enalapril, compared to enalapril alone.14 Furthermore, Fisman et al. reported that treatment with aspirin 500 mg/day for one week did not influence BP control in hypertensive or in post-infarction patients receiving an ACEI.83 Also of note is the fact that combined treatment with aspirin (100 and/or 300 mg/day) and ACEIs reduced the ACEI-induced increased in PRA.10,75
The interaction of aspirin with ARBs does not appear to negatively influence BP control. No significant BP differences were observed when losartan was associated with aspirin therapy (81 mg or 325 mg/day) for two weeks.14 Of note, a post-hoc subgroup analysis of the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study revealed that the combination of aspirin with losartan exerted greater cardiovascular protection than the combination of aspirin with atenolol, despite similar BP reduction.15 Additionally, a significant decrease in cardiovascular events was found in patients using aspirin plus losartan, compared with losartan alone.15 Interestingly, Prikryl et al. showed that the addition of aspirin to telmisartan improved the effects of this ARB on BP, heart rate, intrarenal resistive index and ejection fraction.84
There have also been large and medium- or long-term trials assessing the BP effects of aspirin in patients on antihypertensive monotherapy or combination therapy (Table 2). Avanzini et al. reported that there was no significant change in BP after addition of aspirin (100 mg/day) to antihypertensive therapy.13 Results from the Hypertension Optimal Treatment (HOT) randomized trial also revealed that there were no differences in achieved BP in patients randomized to aspirin or placebo.4 Importantly, the combination of aspirin with antihypertensive treatment reduced the risk of acute myocardial infarction without increasing the risk of cerebral bleeding.4 Furthermore, the BP values achieved, the intensity of the antihypertensive regimen, and the number and type of drugs used were very similar in aspirin and placebo patients, not only in the overall HOT study population, but in all subgroups.85 Of note, Leinonen et al. conducted a large observational study to assess the effect of aspirin treatment (50-250 mg/day) on BP of hypertensive patients receiving antihypertensive monotherapy or combination therapy and observed that aspirin reduced DBP regardless of the antihypertensive therapeutic scheme.22
Interaction of aspirin with antihypertensive drugs in hypertensive patients.
| Study | Study design | No. of patients | No. of patients taking aspirin | Age (years) | Aspirin dose | Duration of aspirin treatment | Antihypertensive treatment/dose | Effect of combined aspirin treatment | Other effects |
|---|---|---|---|---|---|---|---|---|---|
| Smith et al.81 | Double-blind, randomized, crossover study | 15 | 15 | 53.3±10.6 (mean ± SD) | 75 mg/day | 2 weeks | Captopril 25 mg every 12 hours | No significant change in MAP (office BP) | Treatment with captopril+aspirin ↓ serum (but not urinary) TXB2 No significant linear correlation of serum TXB2 with MAP |
| Magagna et al.75 | Randomized, placebo controlled, double-blind, two-period crossover trial | 30 (20 treated hypertensive patients) | 20 | 45.7±4.5 (total, mean ± SEM) 41.5±3.9 (atenolol group) 47.8±5.0 (captopril group) | 100 mg/day | 4 weeks | Atenolol 100 mg/day Captopril 50 mg/day | No significant change in office BP | Aspirin significantly ↓ serum and urinary TXB2 in captopril- and atenolol-treated patients (vs. baseline and placebo) Aspirin significantly ↓ PRA in captopril- but not in atenolol-treated patients Aspirin slightly (though not significantly) ↓ urinary 6-keto-PGF1α (PGI2 metabolite) values in both captopril and atenolol groups |
| Polónia et al.82 | Randomized crossover study | 18 | 18 | 52.6±1.9 (mean ± SEM) | 100 mg/day Taken in the morning | 2 weeks | Nifedipine (30 mg) or enalapril (20 mg)/day for 4 weeks (in responders to antihypertensive therapy) Nifedipine (60 mg) or enalapril (40 mg) for 4 weeks (in non-responders at the 4th week of the 1st treatment period) | No effect on office BP in nifedipine- or enalapril-treated patients | |
| Guazzi et al.10 | Randomized, placebo-controlled, crossover study | 52 (26 with mild hypertension, 26 with severe hypertension) | 52 | 54±8 (mean ± SD) | 100 mg/day or 300 mg/day | 5 days | Enalapril 20 mg/day | Aspirin 100 mg had no effect on office BP, either alone or in combination with enalapril Aspirin 300 mg ↓ the efficacy of enalapril by 63% in mild hypertensives and by 91% in severe hypertensives | In aspirin 300 mg responders (i.e. with more than 20% attenuation of enalapril's antihypertensive effect), the enalapril-induced rise in PRA was significantly inhibited |
| Hansson et al.4 | Prospective, randomized, open with blinded endpoint evaluation (PROBE) design (HOT randomized trial) | 18790 (491 lost to follow-up) | 9399 (245 lost to follow-up in aspirin group) (246 of 9391 patients assigned to placebo group lost to follow-up) | 61.5 (mean) for those remaining in the study 61.3 (mean) for those lost to follow-up | 75 mg/day | 3.8 years (mean) | Step 1: Felodipine 5 mg/day given to all patients (additional therapy and dose increments in further steps to reach the randomized target BP) Step 2: ACEI or BB added Step 3: felodipine 10 mg once a day Step 4: doubling the dose of either the ACEI or the BB Step 5: diuretic added | No differences in achieved BP (office BP) in patients randomized to aspirin or placebo | Association of aspirin with antihypertensive treatment ↓ the risk of acute myocardial infarction without raising the risk of cerebral bleeding |
| Nawarskas et al.14 | Double-blind, placebo-controlled, partial crossover | 17 | 17 | 44±8.9 (enalapril group, n=7, mean ± SD) 47±13.2 (losartan group, n=10, mean ± SD) | 81 mg/day or 325 mg/day | 2 weeks | Enalapril ≥5 mg/day Losartan ≥25 mg/day | No significant change in MAP, SBP or DBP (office BP) for both aspirin doses in both enalapril and losartan groups | ↓ serum TXB2 in both enalapril and losartan groups treated with aspirin Complete suppression of TXB2 in response to aspirin 325 mg |
| Avanzini et al.13 | Randomized, controlled, open, 2×2 factorial trial (conducted in the framework of the PPP) | 142 | 71 | 59.0±6.0 (total, mean ± SD) 58.9±6.4 (aspirin) 59.2±5.6 (control) | 100 mg/day | 3 months | ACEIs (51.1% of patients) Calcium antagonists (48.2%) BBs (29.8%) Diuretics (18.4%) Alpha-blockers (14.2%) ARBs (6.4%) Centrally acting agents (3.5%) Drugs and dose not specified | No change in office BP or in 24-hour BP (on ABPM) compared to baseline Similar BP pattern in control group | |
| Fisman et al.83 | Self-matched control study | 31 non-smoking hypertensive or post-infarction patients with ACEI- induced cough | 31 | 61±0.9 | 100 mg/day followed by 500 mg/day | 1 week (100 mg/day) +1 week (500 mg/day) | ACEIs Drug and dose not specified | Aspirin did not influence BP control in hypertensives or in post-infarction patients, regardless of dose | Aspirin 100 mg had no effect on ACEI-induced cough Aspirin 500 mg markedly ↓ ACEI-induced cough in the majority of patients |
| Zanchetti et al.85 | Post-hoc subgroup analysis of HOT trial | 18 790 (491 lost to follow-up) | 9399 (245 lost to follow-up in aspirin group) (246 of 9391 patients assigned to placebo group lost to follow-up) | 61.5 (mean) for those remaining in the study 61.3 (mean) for those lost to follow-up | 75 mg/day | 3.8 years | Step 1: Felodipine 5 mg/day given to all patients (additional therapy and dose increments in further steps to reach the randomized target BP) Step 2: ACEI or BB added Step 3: felodipine 10 mg once a day Step 4: doubling the dose of either the ACEI or the BB Step 5: diuretic added | The SBP and DBP (office BP) values achieved, the intensity of the antihypertensive regimen, and the number and type of drug used were similar in aspirin and placebo patients, not only in the overall HOT Study population, but in all subgroups | |
| Fossum et al.15 | Subgroup post-hoc analysis of LIFE study | 9193 | 1970 with aspirin at baseline (1004 in the losartan group; 966 in the atenolol group) (7223 without aspirin) | 68.4±6.7 (mean ± SD, aspirin at baseline) 66.6±7.0 (mean ± SD, without aspirin at baseline) | Aspirin dose not specified (probably low-dose used for prevention of CV events) | 4.7 years | Losartan 50 mg or 100 mg with or without additional antihypertensive drugs Atenolol 50 mg or 100 mg with or without additional drugs | Office BP was similarly ↓ in aspirin+losartan or aspirin+atenolol patients | Greater ↓ in CV death, stroke, and myocardial infarction with losartan-based compared to atenolol-based treatment in patients using aspirin (vs. patients not using aspirin at baseline) |
| Prikryl et al.84 | Crossover, double-blind, randomized trial | 20 | 20 | 46.7±8.6 (mean ± SD) | 100 mg/day | 7 days, each day at a different circadian stage (on awakening, i.e., 06:00, and 3, 6, 9, 12, 15, and 18 hours after awakening) | Telmisartan 80 mg/day | Addition of aspirin to telmisartan further ↓ circadian amplitude of SBP and DBP (office BP) | Addition of aspirin to telmisartan further ↓ heart rate amplitude and intrarenal resistive index; it also caused a slight ↑ in ejection fraction |
| Leinonen et al.22 | Observational | 905 | 246 | 65.5±10.9 (total, mean ± SD) 69.4±9.4 (aspirin group) 64.1±11.1 (no aspirin group) | 50-250 mg/day | Aspirin given either on the day of measurement or at least 3 times during the preceding week | Antihypertensive monotherapy (41% aspirin vs. 47.5% no aspirin): - ACEIs (9.8% vs. 14.6%) - ARBs (1.6% vs. 4.2%) - BBs (15.4% vs. 12.0%) - Calcium antagonists (8.1% vs. 9.1%) - Central acting drugs (0% vs. 0.3%) - Diuretics (6.1% vs. 7.3%) - Combination therapy (58.9% vs. 52.6%) Drugs and dose not specified | Aspirin-treated patients had ↓ DBP and MAP (office BP) Aspirin ↓ DBP regardless of whether patients were on antihypertensive monotherapy or combination therapy In univariate linear regression, the use of aspirin was associated with ↓ DBP and MAP In stepwise multiple linear regression, the use of aspirin remained a significant independent predictor of ↓ DBP | |
| Dimitrov et al.86 | Randomized, open-label, crossover trial | 75 with long-standing hypertension (12±10 years of hypertension, mean ± SD) | 75 | 65±9 | 75 mg/day (64% of patients) 80-160 mg/day (33% of patients) 300 mg/day (3% of patients) | One group (n=39) taking aspirin for 1 month in the evening, followed by 1 month in the morning Another group (n=36) taking aspirin for 1 month in the morning, then 1 month in the evening | One antihypertensive drug - 13 patients Two drugs - 17 patients Three drugs - 26 patients Four drugs - 12 patients Five drugs - 6 patients Six drugs - 1 patient Drugs and doses not specified | The time of aspirin administration did not significantly modify 24-hour SBP or 24-hour DBP, or diurnal or nocturnal SBP and DBP (ABPM) values | |
| Bonten et al.87 | Prospective, randomized, open-label, blinded endpoint (PROBE), 2-period crossover study | 290 with CV disease (263 in the final analysis) | 290 (263 in the final analysis) | 64±7 (mean ± SD) | 100 mg/day | One group (n=134) taking aspirin on awakening for 3 months, followed by aspirin at bedtime for the same period Another group (n=129) taking aspirin at bedtime for 3 months, followed by aspirin on awakening for the same period | BBs (51% in the awakening-bedtime group vs. 55% in the bedtime-awakening group) ACEIs (41% vs. 38%) ARBs (26% vs. 23%) Calcium antagonists (20% vs. 19%) Diuretics (26% vs. 32%) Drugs and doses not specified. | Aspirin intake at bedtime did not ↓ BP compared with intake on awakening | Bedtime aspirin significantly ↓ morning platelet reactivity compared to aspirin on awakening |
↑: increase[d]; ↓: decrease[d]; ABPM: ambulatory blood pressure measurement; ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; BBs: beta-blockers; BP: blood pressure; CV: cardiovascular; DBP: diastolic blood pressure; HOT: Hypertension Optimal Treatment; LIFE: Losartan Intervention For Endpoint reduction in hypertension; MAP: mean arterial pressure; PGI2: prostacyclin; PRA: plasma renin activity; PPP: Primary Prevention Project; SBP: systolic blood pressure; SD: standard deviation; SEM: standard error of the mean; TXB2: thromboxane B2.
The chronotherapeutic effects of aspirin have also been investigated in treated hypertensive patients (Table 2). The administration time-dependent effects of aspirin were assessed in patients with long-standing hypertension and treated with aspirin for cardiovascular prevention. Hypertensive patients were randomly assigned to take aspirin (75-160 mg/day) in the morning followed by aspirin in the evening, or in the reverse order, during two one-month periods, with no washout period. No differences were found in mean 24-hour SBP or DBP, or in patients’ dipper/non-dipper status, independently of aspirin administration time.86 The timing effect of aspirin dosing on BP was further studied in a recent crossover trial including patients taking low-dose aspirin for secondary cardiovascular disease prevention. Subjects were randomized to receive aspirin on awakening then at bedtime, or the reverse order, during two three-month periods, with no washout period. No differences were found in mean 24-hour SBP or DBP when aspirin was administered at awakening or at bedtime. However, morning platelet reactivity was significantly reduced when aspirin was taken at bedtime.87 These results86,87 contradict those observed for bedtime aspirin administration in patients with untreated mild hypertension or those at risk of developing hypertension,70–73,77–80 and may be explained by differences in study populations or by a weakened chronopharmacological effect of aspirin in patients taking this drug for a long time.86,87 A summary of studies assessing aspirin's effects in treated hypertensive patients is presented in Table 2.
ConclusionsBesides its well-known antithrombotic effect, aspirin exerts protective effects on redox status and vascular reactivity and triggers the synthesis of SPMs from AA and omega-3 fatty acids, which may account for its overall cardiovascular benefits.
Most studies assessing aspirin's effects on BP in experimental and human hypertension indicate that, when used in low doses, aspirin by itself does not affect BP values and does not counteract the BP-lowering efficacy of antihypertensive drugs. Of note, combined treatment with ARBs and aspirin appears to improve cardiovascular protection, compared to ARBs alone. Nevertheless, experimental studies assessing aspirin's interaction with antihypertensive drugs are scarce and have done little to identify the mechanisms underlying its protective or deleterious effects.
In subjects with long-standing hypertension and treated with antihypertensive drugs, the time of aspirin administration does not seem to affect BP control, although bedtime intake might exert stronger protection against cardiovascular events due to its significant effect in lowering morning platelet reactivity.
In conclusion, low doses of aspirin do not negatively influence BP control by antihypertensive drugs and appear to enhance cardiovascular protection when associated with ARBs. Further studies are needed to elucidate the mechanisms responsible for these effects.
FundingThis work was supported by FEDER funds via COMPETE and by national funds through FCT – Portuguese Foundation for Science and Technology (project grants: PTDC/SAU-TOX/114166/2009 and EXPL/IVC-PEC/1302/2013). Teresa Sousa was also funded by FCT (SFRH/BPD/112005/2015).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.








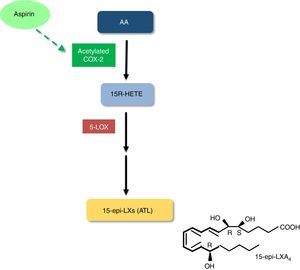 5-LOX: 5-lipoxygenase;
5-LOX: 5-lipoxygenase; 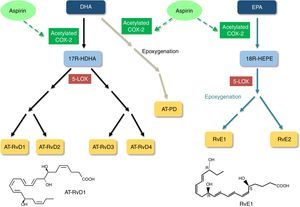 5-LOX: 5-lipoxygenase;
5-LOX: 5-lipoxygenase; 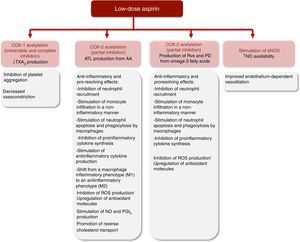 ATL: aspirin-triggered lipoxin;
ATL: aspirin-triggered lipoxin; 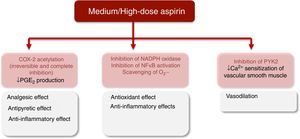 COX-2: cyclooxygenase-2; NF-κB: nuclear factor kappa B;
COX-2: cyclooxygenase-2; NF-κB: nuclear factor kappa B; 

