To characterize the prevalence and clinical correlates of anti-troponin I antibodies in renal transplant patients.
MethodsA group of 48 consecutive renal transplant patients under immunosuppressive therapy were studied. Anti-troponin I antibodies were measured and clinical data were retrieved.
ResultsAn anti-troponin I antibody titer <1:40 was seen in most patients (30). IgG antibody titers ≥1:80 were seen in eight patients, with a single value of 1:160. Regarding IgM antibodies, in six cases titers ≥1:80 were seen, with one value of 1:320. In only one patient were both anti-troponin I antibody IgG and IgM titers 1:80 or higher. Clinical cardiac disease was seen in nine patients. The presence of an anti-troponin I antibody titer ≥1:80 was not associated with the presence of clinical cardiac disease (p=0.232), but was associated with statin therapy status (p=0.008), being less frequent in patients under statin therapy.
ConclusionsAnti-troponin I antibodies are seen in a minority of renal transplant patients, and are not associated with the presence of clinical heart disease, but are associated with lack of statin therapy.
Caracterizar a prevalência e os aspetos clínicos associados à presença de anticorpos antitroponina I em doentes com transplante renal.
MétodosFoi estudado um grupo de 48 doentes consecutivos com transplante renal sob terapêutica imunossupressora. Os anticorpos antitroponina I foram medidos e os dados clínicos foram obtidos.
ResultadosUm título <1:40 de anticorpos antitroponina I foi encontrado na maioria dos doentes (30 doentes). Os anticorpos IgG com títulos ≥1:80 foram encontrados em 8 doentes, com um único valor de 1:160. No caso dos anticorpos IgM, em seis casos os títulos foram ≥1:80, com um caso de título de 1:320. Apenas num doente foram os títulos dos anticorpos antitroponina I de tipo IgG e IgM simultaneamente ≥1:80. A doença cardíaca clínica existia em nove doentes. A presença de anticorpos antitroponina I com títulos ≥1:80 não se associou com a presença de doença cardíaca clínica (nível de significância 0,232), mas associou-se com a ausência de terapêutica com estatina (nível de significância 0,008).
ConclusõesOs anticorpos antitroponina I encontram-se presentes numa minoria de doentes com transplante renal, não se verificando uma associação com a presença de doença cardíaca clínica, mas com uma associação com ausência de terapêutica com estatina.
Patients with chronic renal disease, including those on hemodialysis, have a high mortality rate, and a significant proportion of this mortality is due to cardiovascular disease.1,2 Renal transplantation may lead to better long-term survival than hemodialysis.3
Antibodies to cardiac troponin I are elevated in a significant number of patients with both ischemic cardiomyopathy and idiopathic dilated cardiomyopathy.4,5 Absence of autoantibodies against cardiac troponin I was found in one study to predict improvement of left ventricular function after acute myocardial infarction.5 In another study, however, the presence of the same type of autoantibodies in plasma was associated with improved survival in patients with chronic dilated cardiomyopathy, but not with ischemic cardiomyopathy.6
We recently described a case of hemodialysis-associated dilated cardiomyopathy with a marked improvement in left ventricular ejection fraction (LVEF) after renal transplantation, in association with high titers of anti-troponin I antibodies.7
In the present study, anti-troponin I antibodies were measured in a group of consecutive renal transplant patients, in order to characterize the pattern of these antibodies in this type of patients, and also to correlate anti-troponin I antibodies with the cardiac features of the patients under study.
MethodsThe study protocol was approved by the institutional ethics committee, and the patients gave their written consent. A total of 48 consecutive patients with chronic renal failure and renal transplant were admitted to the study. Peripheral venous blood samples were collected from all patients, at the same time as collecting routine blood samples (during November and December 2013), and serum samples were frozen. Anti-troponin I antibodies (IgG and IgM) were measured as previously described,5 blinded from patients’ clinical data. Clinical assessment and management of patients was also carried out blinded to the present study.
Clinical data, as well as data from cardiac tests, were retrieved from each patient file. All patients had echocardiographic and electrocardiographic data available, allowing assessment of left ventricular systolic function, as well as cardiac rhythm. Additional cardiac data were available for some patients.
Data on the renal status of each patient were also retrieved from the files, including date of transplant, etiology of renal failure, current renal function and current immunosuppressive therapy.
Associations between the clinical data of interest and the presence of anti-troponin I antibodies with a titer ≥1:80 were analyzed by the chi-square test (SPSS version 22 software, IBM, Armonk, NY), with a significance level of p<0.05.
ResultsThe mean age of the patients was 52.9±12.6 years; 18 were female and 30 male, and all were Caucasian.
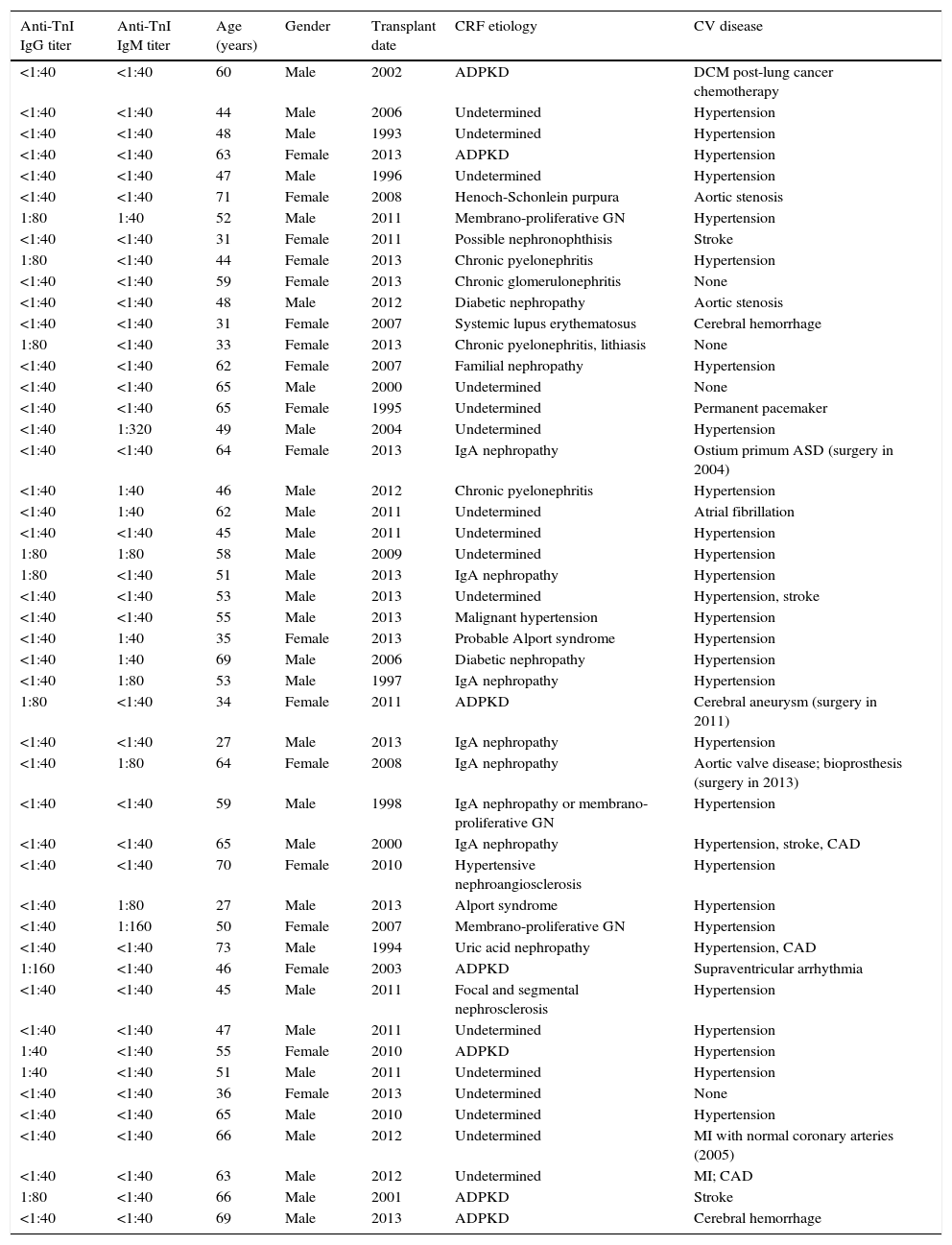
The main results are presented in Table 1. The etiology of chronic renal failure was varied, and undetermined in a significant number of cases, sometimes due to inconclusive renal biopsy. Autosomal dominant polycystic kidney disease (ADPKD) was present in seven cases, and IgA nephropathy was also a relatively frequent diagnosis.
Main data on renal transplant patients (n=48).
| Anti-TnI IgG titer | Anti-TnI IgM titer | Age (years) | Gender | Transplant date | CRF etiology | CV disease |
|---|---|---|---|---|---|---|
| <1:40 | <1:40 | 60 | Male | 2002 | ADPKD | DCM post-lung cancer chemotherapy |
| <1:40 | <1:40 | 44 | Male | 2006 | Undetermined | Hypertension |
| <1:40 | <1:40 | 48 | Male | 1993 | Undetermined | Hypertension |
| <1:40 | <1:40 | 63 | Female | 2013 | ADPKD | Hypertension |
| <1:40 | <1:40 | 47 | Male | 1996 | Undetermined | Hypertension |
| <1:40 | <1:40 | 71 | Female | 2008 | Henoch-Schonlein purpura | Aortic stenosis |
| 1:80 | 1:40 | 52 | Male | 2011 | Membrano-proliferative GN | Hypertension |
| <1:40 | <1:40 | 31 | Female | 2011 | Possible nephronophthisis | Stroke |
| 1:80 | <1:40 | 44 | Female | 2013 | Chronic pyelonephritis | Hypertension |
| <1:40 | <1:40 | 59 | Female | 2013 | Chronic glomerulonephritis | None |
| <1:40 | <1:40 | 48 | Male | 2012 | Diabetic nephropathy | Aortic stenosis |
| <1:40 | <1:40 | 31 | Female | 2007 | Systemic lupus erythematosus | Cerebral hemorrhage |
| 1:80 | <1:40 | 33 | Female | 2013 | Chronic pyelonephritis, lithiasis | None |
| <1:40 | <1:40 | 62 | Female | 2007 | Familial nephropathy | Hypertension |
| <1:40 | <1:40 | 65 | Male | 2000 | Undetermined | None |
| <1:40 | <1:40 | 65 | Female | 1995 | Undetermined | Permanent pacemaker |
| <1:40 | 1:320 | 49 | Male | 2004 | Undetermined | Hypertension |
| <1:40 | <1:40 | 64 | Female | 2013 | IgA nephropathy | Ostium primum ASD (surgery in 2004) |
| <1:40 | 1:40 | 46 | Male | 2012 | Chronic pyelonephritis | Hypertension |
| <1:40 | 1:40 | 62 | Male | 2011 | Undetermined | Atrial fibrillation |
| <1:40 | <1:40 | 45 | Male | 2011 | Undetermined | Hypertension |
| 1:80 | 1:80 | 58 | Male | 2009 | Undetermined | Hypertension |
| 1:80 | <1:40 | 51 | Male | 2013 | IgA nephropathy | Hypertension |
| <1:40 | <1:40 | 53 | Male | 2013 | Undetermined | Hypertension, stroke |
| <1:40 | <1:40 | 55 | Male | 2013 | Malignant hypertension | Hypertension |
| <1:40 | 1:40 | 35 | Female | 2013 | Probable Alport syndrome | Hypertension |
| <1:40 | 1:40 | 69 | Male | 2006 | Diabetic nephropathy | Hypertension |
| <1:40 | 1:80 | 53 | Male | 1997 | IgA nephropathy | Hypertension |
| 1:80 | <1:40 | 34 | Female | 2011 | ADPKD | Cerebral aneurysm (surgery in 2011) |
| <1:40 | <1:40 | 27 | Male | 2013 | IgA nephropathy | Hypertension |
| <1:40 | 1:80 | 64 | Female | 2008 | IgA nephropathy | Aortic valve disease; bioprosthesis (surgery in 2013) |
| <1:40 | <1:40 | 59 | Male | 1998 | IgA nephropathy or membrano-proliferative GN | Hypertension |
| <1:40 | <1:40 | 65 | Male | 2000 | IgA nephropathy | Hypertension, stroke, CAD |
| <1:40 | <1:40 | 70 | Female | 2010 | Hypertensive nephroangiosclerosis | Hypertension |
| <1:40 | 1:80 | 27 | Male | 2013 | Alport syndrome | Hypertension |
| <1:40 | 1:160 | 50 | Female | 2007 | Membrano-proliferative GN | Hypertension |
| <1:40 | <1:40 | 73 | Male | 1994 | Uric acid nephropathy | Hypertension, CAD |
| 1:160 | <1:40 | 46 | Female | 2003 | ADPKD | Supraventricular arrhythmia |
| <1:40 | <1:40 | 45 | Male | 2011 | Focal and segmental nephrosclerosis | Hypertension |
| <1:40 | <1:40 | 47 | Male | 2011 | Undetermined | Hypertension |
| 1:40 | <1:40 | 55 | Female | 2010 | ADPKD | Hypertension |
| 1:40 | <1:40 | 51 | Male | 2011 | Undetermined | Hypertension |
| <1:40 | <1:40 | 36 | Female | 2013 | Undetermined | None |
| <1:40 | <1:40 | 65 | Male | 2010 | Undetermined | Hypertension |
| <1:40 | <1:40 | 66 | Male | 2012 | Undetermined | MI with normal coronary arteries (2005) |
| <1:40 | <1:40 | 63 | Male | 2012 | Undetermined | MI; CAD |
| 1:80 | <1:40 | 66 | Male | 2001 | ADPKD | Stroke |
| <1:40 | <1:40 | 69 | Male | 2013 | ADPKD | Cerebral hemorrhage |
ADPKD: autosomal dominant polycystic kidney disease; anti-TnI: anti-troponin I antibody; ASD: atrial septal defect; CAD: coronary artery disease; CV: cardiovascular; CRF: chronic renal failure; DCM: dilated cardiomyopathy; GN: glomerulonephritis; MI: myocardial infarction.
Most patients had arterial hypertension. Concerning clinical cardiac disease, four patients had a history of myocardial infarction and/or angiographic coronary artery disease, three had aortic valve disease, one had surgically corrected ostium primum atrial septal defect and one had dilated cardiomyopathy associated with lung cancer chemotherapy. All but one patient (with atrial fibrillation) were in sinus rhythm. Pre-transplant cardiac data were available for some patients, but there were no cases of pre-transplant dilated cardiomyopathy.
With regard to anti-troponin I antibodies, a titer <1:40 was seen in most patients (30). IgG antibody titers of 1:80 or more were detected in eight patients, with a single value of 1:160, in a patient with ADPKD. Regarding IgM antibodies, in six cases titers were higher than 1:40, with one value of 1:320. In only one patient were both anti-troponin I antibody IgG and IgM titers 1:80 or more. A titer of 1:40 was seen in a further four patients in the case of IgM antibodies, and in two in the case of IgG antibodies.
Nine patients had clinical cardiac disease, eight with undetectable anti-troponin I antibodies, while one patient had a 1:80 IgM antibody titer. Analysis by the chi-square test of the association between an anti-troponin I antibody titer ≥1:80 and the presence of clinical cardiac disease yielded p=0.232.
Patients were under double or triple immunosuppressive therapy with prednisolone (45 patients), cyclosporine (nine), tacrolimus (34), everolimus (five), mycophenolate mofetil (37), and mycophenolic acid (three). Statins (3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors) were used by 26 patients. Doses used were in the 10–20 mg range for pravastatin, simvastatin and atorvastatin, except in one case of simvastatin (40 mg). A 5-mg dose of rosuvastatin was used by some patients. Analysis by the chi-square test of the association between anti-troponin I antibodies and statin therapy status yielded p=0.008, corresponding to antibody titers of ≥1:80 in 10/22 patients not under statin therapy, compared to 3/26 patients under statin therapy. The same analysis regarding the association between anti-troponin I antibodies and each immunosuppressive drug yielded non-significant results in each case (data not shown).
DiscussionIn the present study, we describe levels of anti-troponin I antibodies in a series of renal transplant patients. Most had low anti-troponin I antibody titers (1:40 or lower). It is not known whether immunosuppressive therapy can alter the titers for anti-troponin I antibodies.
One patient had dilated cardiomyopathy associated with lung cancer chemotherapy, and all other patients had preserved LVEF. Eight additional patients had clinical heart disease and one had atrial fibrillation. We could find no association between the presence of clinical heart disease and the presence of anti-troponin I antibodies.
A reversible cardiomyopathy is found in a significant number of patients with end-stage renal disease, and LVEF increases in some of these patients after renal transplantation.8,9 As stated above, we recently described a case of hemodialysis-associated dilated cardiomyopathy with a marked improvement in LVEF after renal transplantation, in association with high titers of anti-troponin I antibodies.7 However, there appears to be no such case in this series of patients.
An association was seen between the presence of anti-troponin I antibodies with a titer of 1:80 or higher and the absence of statin therapy. We may speculate that statins have an inhibitory effect on the production of anti-cardiac, in this case anti-troponin I, antibodies. In fact, while statins have marked effects on cardiovascular outcomes in both primary and secondary prevention, similar effects have not been seen with some other types of drugs that decrease low-density lipoprotein cholesterol,10 indicating that additional mechanisms could be important in the actions of statins, one of which could be the possible effect mentioned above. Statins are believed to have immunomodulatory effects, including effects on major histocompatibility complex class II molecules and T helper cells,11 as well as on leukocyte function-associated antigen-1.12 Statin treatment has been associated with reduced heat shock protein antibody titers in patients with dyslipidemia.13 The association now reported could represent a form of indication bias – patients with dyslipidemia, and therefore with indication for statin therapy, could be less prone to the presence of anti-troponin I antibodies – or even a mere chance association. In the report by Leuschner et al., the group of 108 patients with acute myocardial infarction were all treated with statins, but 10 patients had a anti-troponin I titer ≥1:160.5 Further studies are needed to clarify this matter.
Study limitationsThe number of patients studied was relatively small. Clinical data were retrieved from existing clinical records and were not obtained prospectively. No attempt was made to characterize adherence to treatment by the patients under study. Given these limitations, the conclusions of the present report must be seen as preliminary.
ConclusionWe conclude that anti-troponin I antibodies are seen in a minority of patients with renal transplant patients, and are not associated with the presence of clinical heart disease, but are associated with lack of statin therapy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Marta Oliveira and the nursing staff of Hospital São João for their expert technical and nursing assistance.