A 60-year-old man was admitted for ablation of ventricular tachycardia (VT) following anteroseptal myocardial infarction. Cardiac computed tomography (CT) images were processed with ADAS-VT software (Galgo Medical, Barcelona, Spain). Two large zones of septal and anteroapical wall thinning were found, separated by a very small zone of thicker wall.
High-density electroanatomical mapping was performed using CARTO 3 and the PentaRay catheter (Biosense Webster, Diamond Bar, CA). Local abnormal ventricular activity (LAVA) and late potentials were observed between thinned segments.
Two badly tolerated VTs could be induced, VT1 (right bundle branch block, superior axis) and VT2 (left bundle branch block, inferior axis).
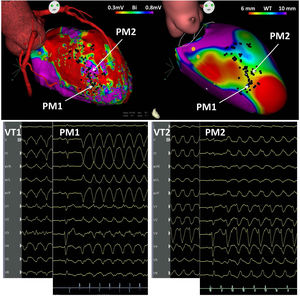
A pace-mapping strategy was pursued. Pace mapping along the area of the 6-10 mm wall thinning zone, between the areas of <6 mm wall thinning, matched both VT morphologies, demonstrating that activation proceeded along the exit or entry parts of the isthmus according to slight changes in the pacing site. VT1 and VT2 shared a common isthmus in the 6-10 mm thickness zone, in opposite directions (Figure 1).
Top left: voltage map of the left ventricle in right anterior oblique view, showing a large zone of scar in the anterior and septal regions, harboring local abnormal ventricular activations (black dots); top right: cardiac computed tomography images processed with ADAS-VT software (Galgo Medical, Barcelona, Spain) showing two large zones of septal and anteroapical wall thinning (<6 mm, red), separated by a very small zone of thicker wall (yellow, 6-10 mm). The morphology of the 12-lead electrocardiogram of VT1, VT2, and PM1 and PM2 is shown in the bottom traces. PM: pace-mapping; VT: ventricular tachycardia.
Ablation was performed targeting LAVA. The patient's VT was eventually non-inducible and there were no recurrences during follow-up.
Integration of magnetic resonance imaging and/or CT scans with electroanatomical maps are associated with better procedural outcomes. Regional myocardial wall thinning correlates with low-voltage zones and abnormal potentials that are critical for the maintenance of VT. Such an approach may help to detect the underlying critical substrate itself and to understand the mechanisms of VT, which should lead to procedural success.
However, the sensitivity and positive predictive value of CT scan to detect VT isthmuses are not yet optimal, and further studies are also warranted to better define the minimal myocardial thickness able to harbor a VT isthmus.
Conflicts of interestThe authors have no conflicts of interest to declare.