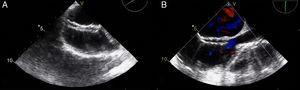
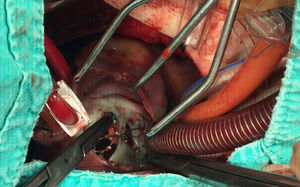
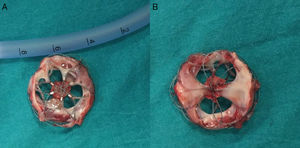
A 21-year-old male with a 27-mm atrial septal defect (ASD) underwent an uneventful percutaneous device closure using a 28-mm Ultrasept ASD Occluder with super-low profile (Cardia, Inc., USA) under transoesophageal echocardiography (TEE) guidance. We particularly favoured this kind of occluder device due to the possible future need for catheter atrial septostomy in such a young patient. The use of TEE during the procedure ensured that the occluder device was properly deployed in the middle part of the interatrial septum and there was no residual shunt through it (Figure 1). There were also no problems with the occluder device at 1-month follow-up. However, four months after the procedure, the patient presented again with vague chest pain, palpitations and shortness of breath. The physical examination was unremarkable. Transthoracic echocardiography (TTE), followed by TEE, revealed a significant left-to-right shunt, although the device was clearly seen in the middle part of the interatrial septum. The initial differential diagnosis included partial displacement of the device and therefore, the patient was referred to surgery, all margins of the occluder device were found to be properly attached to the interatrial septum during the surgery (Figure 2). Interestingly, the central patch of the device had almost completely disappeared (Figure 3). Surgical repair of the atrial septum was performed successfully after removing the metallic part of the device. There were no signs of emboli in the brain or pulmonary vasculature on the computed tomography scan, which was performed following the surgery for any possible embolisation of the patch material. The patient was discharged from the hospital without any complications and in good health.
In conclusion, although device closure of ASDs can safely be performed percutaneously, recanalisation may occur on rare occasions, probably due to device characteristics of the Ultrasept ASD Occluder. While ASD occluder devices from this brand are usually preferred due to their super-low profile, this feature of the devices may not provide necessary support and resistance in the interatrial septum. As a matter of fact, the last generation of Ultrasept ASD Occluder devices have an extra layer between the two other layers, indicating this kind of necessity.
Conflicts of interestThe authors have no conflicts of interest to declare.