To determine whether there are differences in blood pressure profile on dynamic assessment by ambulatory blood pressure monitoring (ABPM) according to serum sodium levels in stable heart failure patients.
MethodsData were collected from the Spanish National Registry on Ambulatory Blood Pressure Monitoring in Heart Failure (DICUMAP). Patients underwent ABPM by the oscillometric principle using a Spacelabs 90121 monitor. The sample was divided into three groups according to sodium levels and their clinical and laboratory data and echocardiographic findings were analyzed. Robust statistical methods were used to compare the groups in univariate and multivariate models.
ResultsA total of 175 patients (44.57% male) were analyzed. We found a predominance of anomalous circadian blood pressure profiles in all three groups, with a significantly higher percentage of risers in the lowest serum sodium group (p=0.05). In addition, in this group there were significant differences in mean 24-hour systolic blood pressure (SBP) (24-h SBP, p=0.05) and in mean daytime SBP (dSBP, p=0.008), with significant differences in nocturnal fall in SBP (p=0.05) and in diastolic blood pressure (p=0.005). In multivariate analysis a significant relationship was found between sodium levels and 24-h SBP (OR 0.97, 95% CI 0.95-0.99, p=0.01) and dSBP (OR 0.96, 95% CI 0.94-0.99, p=0.004).
ConclusionA relationship was found between lower sodium levels and lower systolic blood pressure, especially during waking hours, with a lower decline between daytime and night-time blood pressure.
Determinar se existem diferenças no perfil da pressão arterial, através da monitorização da pressão ambulatória da pressão arterial como método de avaliação dinâmica nos doentes estáveis com insuficiência cardíaca, dependendo dos níveis de sódio sérico.
MétodosOs dados do doente foram obtidos do Registo Nacional Espanhol para Insuficiência Cardíaca e Medição Ambulatória da Pressão Arterial. Submeteram-se à monitorização ambulatória da pressão arterial através do princípio oscilométrico, utilizando um monitor Spacelabs 90121. Dividimos a amostra em três grupos de acordo com os níveis de sódio e analisámos os dados clínicos e laboratoriais, bem como os achados ecocardiográficos. Utilizámos metodologia estatística consistente para comparar os grupos em modelos univariados e multivariados.
ResultadosForam analisados 175 doentes (44,57%). Relativamente à pressão arterial, constatámos a predominância do perfil circadiano anómalo nos três grupos, com uma percentagem significativa mais elevada de um perfil de elevação no grupo com sódio sérico mais baixo (p=0,05). Além disso, constatámos neste grupo diferenças significativas nos valores médios da pressão arterial sistólica em 24 h (PAS24 h, p=0,05) e nos valores médios da pressão arterial sistólica durante período vígil (PASd, p=0,008), com uma diferença significativa na descida noturna da PAS (p=0,05) e da pressão arterial diastólica (p=0,005). Numa análise multivariada ajustada, constatámos uma relação significativa entre os níveis de sódio e a PAS24 h (OR 0,97, IC 95% 0,95-0,99, p=0,01) e a PASd (OR 0,96, IC 95% 0,94-0,99, p=0,004).
ConclusãoConstatámos uma relação entre os níveis mais baixos de sódio e a PAS mais baixa, especialmente durante o período vígil, com uma descida decrescente entre a pressão arterial diurna e noturna.
Hyponatremia is a common complication in heart failure (HF), especially when the disease is advanced. Studies in various patient populations, ranging from acutely decompensated hospitalized patients to stable outpatients, have shown an association between low serum sodium concentrations and poor prognosis.1–3 Equally, risk models developed in HF cohorts of outpatients4 and hospitalized patients5,6 have suggested that hyponatremia is one of the most powerful predictors of mortality. Blood pressure (BP) is another predictor of survival,7 and lower sodium concentrations are often associated with low BP. However, this association has not been thoroughly studied and is poorly understood, due in part to the lack of studies that provide dynamic 24-hour BP measurement.
The influence of hyponatremia on BP in HF patients may be the result of changes in both plasma sodium levels and extracellular volume (ECV), but may also be secondary to antihypertensive drugs used to treat HF. It may therefore be useful to know whether BP remains low at night or whether these patients’ circadian profile is disrupted.
Our aim in this study was to determine whether there are differences in BP behavior on dynamic assessment by ambulatory blood pressure monitoring (ABPM) in stable HF patients with different serum sodium levels.
MethodsPatientsData were collected from the Spanish National Registry on Ambulatory Blood Pressure Monitoring in Heart Failure (DICUMAP) of the Spanish Society of Internal Medicine. This was a multicenter prospective cohort study that ran from 2009 to 2013 and included data from 17 Spanish hospitals. The study complied with the principles outlined in the Declaration of Helsinki, the study protocol was approved by an ethics committee, and informed consent was obtained from all patients prior to inclusion in the registry. Inclusion criteria were age >40 years, with stable HF diagnosed according to the Framingham criteria,8 and with hypertensive, ischemic, or idiopathic cardiomyopathy as the primary cause of HF. Exclusion criteria were HF due to valvulopathy, acute HF decompensation (more than 15 days from discharge) and contraindications to ABPM (uncontrolled atrial fibrillation or other tachyarrhythmia, night work and arm circumference greater than 42 cm).
Data were collected through a website (https://www.registrodicumap.org/) which housed the database, accessed with a personal password. Confidentiality was preserved since no personal data were stored except date of birth and initials to avoid data duplication.
Ambulatory blood pressure monitoringPatients underwent ABPM by the oscillometric principle using a Spacelabs 90121 monitor (Spacelabs Inc., WA, USA). Data analysis was performed using Spacelabs 90121 report management software installed on a personal computer. Data were sent to www.registrodicumap.org in ASCII format. The monitor's cuff was placed on the patient's non-dominant arm in the morning and removed at the same time the following morning. The patients were given a diary to record any unexpected events and times of going to sleep and waking, and were instructed to relax the cuffed arm when inflation began. Minimum criteria to validate the recording were more than 60 successful readings out of a total of 95 scheduled, with at least two effective readings per hour.
Data collected from the patients, adjusted to waking and sleeping periods according to patient diaries, included mean 24-h systolic BP (24-h SBP), mean 24-h diastolic BP (24-h DBP), mean daytime systolic BP (dSBP), mean daytime diastolic BP (dDBP), mean night-time systolic BP (nSBP) and mean night-time diastolic BP (nDBP). The differences between dSBP and nSBP (dip-SBP) and between dDBP and nDBP (dip-DBP) were also calculated and the patients were classified according to their circadian BP profile as dipper when BP declined more than 10% from the daytime level, non-dipper when BP declined less than 10% from the daytime level, riser when night-time BP was higher than daytime BP, and extreme dipper when BP declined more than 20% from the daytime level, and also as reducers (dipper and extreme dipper) or non-reducers (non-dipper and riser).
Study variablesThe DICUMAP registry included sociodemographic information, comorbidities, clinical data (BP, heart rate and body mass index [BMI]), and laboratory data including hemoglobin, standard biochemical parameters and natriuretic peptides when available. HF severity was classified by New York Heart Association (NYHA) functional class and left ventricular ejection fraction (LVEF) on two-dimensional echocardiography.
Statistical analysisTo analyze the influence of serum sodium on BP, the study population was divided into quartiles by sodium level and three groups were created by combining the second and third quartiles, to obtain clearly differentiated groups: Group 1, Na 133-139 mEq/l (n=48); Group 2, Na 140-142 mEq/l (n=81); and Group 3, Na 143-147 mEq/l (n=46). A flowchart of the patient selection process is shown in Figure 1.
Robust statistical methods were used which are not unduly affected by outliers or small departures from model assumptions. Qualitative and categorical data were expressed as absolute number and percentage and were compared in univariate analysis by the chi-square test. Quantitative data were expressed as 20% trimmed mean and median absolute deviation (MAD) and were compared in univariate analysis by ANOVA in cases of normality and homoscedasticity with the approximate Welch's t test in cases of unequal variances in the samples, and the Rust-Fligner test in the remainder. Multivariate analysis was performed using Cantoni and Ronchetti's approach9 based on robust quasi-deviance functions for estimation and variable selection. The final model, based on whether patients belonged to Group 1, was adjusted for age, gender, Charlson comorbidity index, BMI, heart rate, estimated glomerular filtration rate (eGFR) and LVEF.
The software package used for the statistical analysis was R version 3.2.1 (2015-06-18).
ResultsA total of 186 patients (44.57% male, mean age 76.88±8.16 years) were included. Their baseline characteristics according to group of serum sodium levels are shown in Table 1. eGFR and blood glucose levels were slightly higher in Group 1, but not significantly (p=0.78 and p=0.26, respectively). Group 2 had a significantly higher percentage of patients with preserved ejection fraction (86.42% vs. 70.83% in Group 1 and 68.89% in Group 3, p=0.03). Brain natriuretic peptide (BNP) was only reported in 39 patients (13 in Group 1, 18 in Group 2 and 8 in Group 3). Although a tendency for higher BP across groups was found, this was not significant (p=0.37).
Baseline characteristics of patients by group of serum sodium levels.
| Variable | Group 1 (n=48) | Group 2 (n=81) | Group 3 (n=46) | p |
|---|---|---|---|---|
| Male | 25 (52.1%) | 35 (43.21%) | 18 (39.13%) | 0.42 |
| Age (years) | 79.35 (7.73) | 77.10 (8.05) | 76.68 (7.31) | 0.38 |
| Hypertension | 45 (83.75%) | 76 (93.83%) | 43 (93.48%) | 0.99 |
| Smoking | 1 (2.08%) | 11 (13.58%) | 5 (10.87%) | 0.09 |
| Alcohol consumption | 14 (29.17%) | 21 (25.93%) | 11 (23.91%) | 0.84 |
| Type 2 diabetes | 22 (45.83%) | 36 (44.44%) | 17 (36.96%) | 0.63 |
| Dyslipidemia | 30 (62.5%) | 42 (51.85%) | 21 (45.65%) | 0.24 |
| CKD | 17 (35.42%) | 28 (34.57%) | 14 (30.43%) | 0.85 |
| Arrhythmia | 25 (52.08%) | 45 (55.56%) | 21 (45.65%) | 0.56 |
| BMI | 29.35 (3.11) | 29.30 (4.31) | 29.38 (3.03) | 0.55 |
| SBP (mmHg) | 131.8 (19.64) | 139.2 (22.23) | 138 (12.60) | 0.1 |
| DBP (mmHg) | 72.48 (14.08) | 74.93 (13.34) | 74.71 (10.74) | 0.8 |
| Heart rate (bpm) | 72.33 (12.60) | 71.83 (11.86) | 73.07 (12.60) | 0.91 |
| Hemoglobin (g/dl) | 12.73 (1.77) | 12.40 (2.22) | 12.87 (1.33) | 0.46 |
| Creatinine (mg/dl) | 1.21 (0.57) | 1.12 (0.44) | 1.06 (0.37) | 0.38 |
| eGFR (ml/min/1.73 m2) | 53.89 (22.97) | 55.48 (25.93) | 57.88 (22.97) | 0.78 |
| Blood glucose (mg/dl) | 117.8 (26.68) | 113.48 (25.2) | 108.46 (20.01) | 0.26 |
| Sodium (mEq/l) | 137.8 (1.48) | 140.97 (1.48) | 143.53 (1.48) | 0.0000 |
| Potassium (mEq/l) | 4.58 (0.57) | 4.44 (0.44) | 4.45 (0.44) | 0.39 |
| BNPa | 416.96 (536.2) | 447.97 (497.4) | 486.52 (411.57) | 0.37 |
| LVEF | 56.23 (14.82) | 60.73 (7.41) | 55.25 (44.08) | 0.0078 |
| Left atrial diameter (n=168) | 39.5 (19.1) | 39.26 (14.47) | 39.90 (11.84) | 0.79 |
| HFpEF | 34 (70.83%) | 70 (86.42%) | 31 (68.89%) | 0.03 |
| NYHA class | ||||
| I | 2 (4.2%) | 12 (14.81%) | 6 (13.04%) | 0.25 |
| II | 35 (72.92%) | 51 (62.96%) | 34 (73.91%) | 0.25 |
| III | 11 (22.92%) | 18 (22.22%) | 6 (13.04%) | 0.25 |
| Etiology | ||||
| Hypertensive | 30 (62.5%) | 62 (76.64%) | 35 (76.09%) | 0.2 |
| Ischemic | 7 (14.5%) | 5 (6.17%) | 6 (13.04%) | 0.2 |
| Both | 11 (22.92%) | 13 (16.05%) | 4 (8.70%) | 0.2 |
| Idiopathic | 0 (0%) | 1 (1.23%) | 1 (2.17%) | 0.2 |
| Treatment | ||||
| Loop diuretics | 34 (70.8%) | 55 (67.9%) | 39 (84.78%) | 0.1 |
| Beta-blockers | 34 (70.8%) | 41 (50.62%) | 28 (60.87%) | 0.07 |
| Thiazides | 8 (16.67%) | 12 (14.81%) | 3 (6.52%) | 0.2 |
| Calcium antagonists | 12 (25%) | 25 (30.86%) | 16 (34.78%) | 0.5 |
| ACEIs/ARBs | 38 (79.2%) | 59 (72.8%) | 38 (82.6%) | 0.41 |
| Aldosterone antagonists | 3 (6.25%) | 4 (4.94%) | 3 (6.52%) | 0.9 |
ACEIs/ARBS: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; BMI: body mass index; BNP: brain natriuretic peptide; CKD: chronic kidney disease; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HFpEF: heart failure with preserved ejection fraction (>45%); LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SBP: systolic blood pressure.
Regarding treatment (Table 1), no differences were found in antihypertensive drugs or diuretics, including thiazides. The proportion of patients in Group 1 taking beta-blockers was slightly higher (70.8% vs. 50.62% in Group 2 and 60.87% in Group 3, p=0.07), although not significantly. Dosages in the total sample were (median and interquartile range): 40 mg (0) for diuretics, 5 mg (7.5) for betablockers, 75 mg (128) for angiotensin receptor blockers, 10 mg (15) for angiotensin-converting enzyme inhibitors, 10 mg (15) for calcium antagonists, 25 mg (0) for aldosterone antagonists and 12.5 mg (12.5) for thiazides.
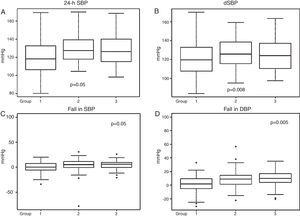
Ambulatory blood pressure monitoringData from 24-h ABPM (Table 2) showed firstly a predominance of anomalous circadian profiles (absence of normal night-time BP fall) in all three groups. In total we found 37.14% non-dippers, 38.29% risers and 4% extreme dippers, without significant differences between the groups. However, Group 1 had a significantly higher percentage with a riser pattern than the other two groups (p=0.05). Secondly, 24-h SBP and dSBP were significantly higher in Groups 2 and 3 (24-h SBP: 118.82±18.8 mmHg in Group 1, 126.39±16.8 mmHg in Group 2 and 123.8±20.1 mmHg in Group 3, p=0.05, and dSBP: 119.37±20.7 mmHg in Group 1, 128.06±16.68 mmHg in Group 2 and 127.18±19.72 mmHg in Group 3, p=0.008). Consequently, significant differences were also found in falls in both SBP and DBP between waking and sleeping times, the smallest fall being seen in Group 1 (Table 2 and Figure 2).
Findings by group, derived from ambulatory blood pressure monitoring.
| Variable | Group 1 (n=48) | Group 2 (n=81) | Group 3 (n=46) | p |
|---|---|---|---|---|
| 24-h SBP (mmHg) | 118.82 (18.88) | 126.39 (16.82) | 123.8 (20.1) | 0.05 |
| 24-h DBP (mmHg) | 64.42 (9.95) | 66.14 (10.32) | 64.52 (13.4) | 0.6 |
| dSBP (mmHg) | 119.57 (20.76) | 128.06 (16.68) | 127.18 (19.72) | 0.008 |
| dDBP (mmHg) | 65.17 (12.12) | 68.75 (9.97) | 67.59 (10.41) | 0.29 |
| nSBP (mmHg) | 117.29 (23.40) | 123.43 (18.11) | 120.22 (19.19) | 0.3 |
| nDBP (mmHg) | 64.28 (11.56) | 62.89 (10.46) | 60.92 (11.02) | 0.37 |
| SBP (mmHg) | 0.21 (9.74) | 4.4 (8.89) | 5.1 (7.41) | 0.5 |
| dip-DBP (mmHg) | 2.14 (9.63) | 8.63 (10.37) | 10.24 (10.15) | 0.16 |
| Circadian profile | ||||
| Dipper | 7 (14.6%) | 18 (22.22%) | 11 (23.91%) | 0.1 |
| Non-dipper | 13 (27.08%) | 32 (39.51%) | 20 (43.48%) | 0.1 |
| Riser | 27 (56.25%) | 26 (32.1%) | 14 (30.43%) | 0.1 |
| Extreme dipper | 1 (2.08%) | 5 (6.17%) | 1 (2.17%) | 0.1 |
| Reducer | 8 (16.67%) | 23 (28.4%) | 12 (26.09%) | 0.3 |
24-h DBP: mean 24-h diastolic blood pressure; 24-h SBP: mean 24-h systolic blood pressure; dDBP: mean daytime diastolic blood pressure; dip-DBP: mean difference between dDBP and nDBP; dip-SBP: mean difference between dSBP and nSBP; dSBP: mean daytime systolic blood pressure; nDBP: mean night-time diastolic blood pressure; nSBP: mean night-time systolic blood pressure.
In multivariate adjusted regression analysis (Table 3), a significant relationship was found between sodium levels and both 24-h SBP (odds ratio [OR] 0.97, 95% confidence interval [CI] 0.95-0.99, p=0.01) and dSBP (OR 0.96, 95% CI 0.94-0.99, p=0.004), but no significant differences in the other variables.
Univariate and multivariate regression analysis of ambulatory blood pressure monitoring variables.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| 24-h SBP | 0.97 | 0.95-0.99 | 0.07 | 0.97 | 0.95-0.99 | 0.01 |
| 24-h DBP | 0.98 | 0.95-1.02 | 0.93 | 0.99 | 0.95-1.03 | 0.62 |
| dSBP | 0.96 | 0.94-0.98 | 0.001 | 0.96 | 0.94-0.99 | 0.004 |
| dDBP | 0.97 | 0.94-1.01 | 0.15 | 0.98 | 0.94-1.02 | 0.31 |
| nSBP | 0.98 | 0.96-1.004 | 0.14 | 0.98 | 0.96-1.00 | 0.064 |
| nDBP | 1.01 | 0.98-1.05 | 0.25 | 1.02 | 0.98-1.05 | 0.29 |
| dip-SBP | 0.98 | 0.96-1.01 | 0.22 | 0.99 | 0.96-1.02 | 0.65 |
| dip-DBP | 0.97 | 0.94-0.99 | 0.03 | 0.97 | 0.95-1.00 | 0.11 |
| Reducer profile | 0.5 | 0.22-1.23 | 0.13 | 0.52 | 0.22-1.25 | 0.14 |
24-h DBP: mean 24-h diastolic blood pressure; 24-h SBP: mean 24-h systolic blood pressure; CI: confidence interval; dDBP: mean daytime diastolic blood pressure; dip-DBP: mean difference between dDBP and nDBP; dip-SBP: mean difference between dSBP and nSBP; dSBP: mean daytime systolic blood pressure; nDBP: mean night-time diastolic blood pressure; nSBP: mean night-time systolic blood pressure; OR: odds ratio.
The main finding in our study is that lower serum sodium levels in outpatients with HF are related to lower SBP, principally during waking hours.
Hyponatremia often coexists with advanced HF,10 and data from registries and clinical trials have shown an association between hyponatremia and increased mortality and morbidity even in outpatients.11 In most of these cases, low BP was linked with low serum sodium, but before the present study, data from a complete 24-h period of BP monitoring had not been published, and previous data were probably based on a single reading per patient, likely to have been taken in the daytime.
In the general population sodium balance is almost entirely controlled by the kidneys through urinary sodium excretion, and the immediate effects of dietary sodium intake are to modify blood sodium and consequently ECV. These changes largely account for later alterations that influence BP, but it is also known that acute rises in sodium intake can trigger a rise in BP by either increasing blood sodium levels even when ECV falls,12 or by increasing ECV when plasma sodium levels decrease.13 This would indicate that acute changes in blood sodium and ECV are both likely able to influence BP independently, which in cases of hyponatremia would lead to lower BP. Although in HF this situation could persist, the reasons are different, particularly in the case of hyponatremia due to upregulation of maladaptive neurohormonal mechanisms, including increased secretion of arginine vasopressin and increased activity of the renin-angiotensin and sympathetic nervous systems, which may underlie the link between development of hyponatremia and HF severity.14 Thus, in HF patients, neurohormonal control may not affect BP as much as in other populations, and reduced cardiac output leading to low SBP may play a more important role; thus, in our study only the differences in SBP were significant, DBP being similar across groups, even though no relationship was found between serum sodium concentrations and LVEF either in our analysis or in most published studies.15–18 In addition, drugs used to treat HF can also influence both BP and hyponatremia. In our study no differences were found between groups regarding the use of these drugs, but it should be noted that in all groups the normal circadian BP profile was absent, with a lower night-time fall in BP, as described previously.19
This lower BP fall while sleeping can be explained by several factors. Firstly, most drugs that affect BP are taken early in the morning, their effects persisting during waking hours but then weakening during the night. Secondly, it could result from a nocturnal increase in central venous pressure as a consequence of dispersion of retained fluids when body position changes during sleep. Thirdly, in healthy individuals the cardiopulmonary baroreflex inhibits sympathetic activation and helps decrease BP, but some studies suggest that impaired baroreflex in HF may cause a reduction in night-time BP decline,20,21 a finding that may also be related to the increased sympathetic stimulation observed in HF, especially in patients with hyponatremia.22 The latter hypothesis could explain the lower nocturnal BP dip in our Group 1, as well as the higher proportion of riser profiles in this group. Reduction in the circadian amplitude of BP rhythm has been related to severity of HF,23 similarly to hyponatremia, and could partly explain the worse outcomes in these patients. It also suggests avenues for further research, focusing on changes in scheduling of medication, administering antihypertensive drugs at the beginning of sleep instead of on waking, aiming to increase night-time BP fall.
Study limitationsThis study has several limitations. Firstly, its results are based on cross-sectional analysis, and so causal relationships cannot be determined. Secondly, because of the small sample size, it was divided by quartiles of sodium levels, instead of comparing a true hyponatremic group (defined as sodium level <135 mEq/l) with other patients; however, some studies have shown worse outcomes when sodium levels drop below 140 mEq/l.1 In addition, the number of patients per group was low, which probably diminished the significance of the results, and so we used robust statistical methods to improve them. On the other hand, the pathogenesis of hyponatremia in HF is multifactorial. Data on osmolarity were not available in all our patients, and thus it is not known whether patients’ hyponatremia was hyper- or hypovolemic. In this regard, diuretic doses were not available by group, but the rate of thiazide use was low, and it therefore seems unlikely that diuretic overuse was the cause of hyponatremia in a significant proportion of cases. Finally, the doses of drugs taken by participants were not assessed, and the lack of this information – which could have influenced BP values – could have biased our results.
On the other hand, our study included the largest number to date of HF patients to undergo ABPM.
ConclusionOur findings show a relationship between lower sodium levels in patients with HF and low SBP, principally during waking hours, and with a smaller fall in BP between daytime and night-time, which could be partly responsible for the worse outcome in these patients. Further studies will be needed to elucidate this subject.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all the investigators of the DICUMAP registry. We are grateful to the DICUMAP Registry Contract Research Organization S&H Medical Science Service for its work in quality control, data management and administrative support.
Investigators of the DICUMAP registry: Miguel Camafort Babkowski; José Carlos Arévalo Lorido; Luis Manzano Espinosa; Javier Sobrino Martínez; José Luis Arias Jiménez; Jorge Francisco Gómez Cerezo; Jesús Díez Manglano; Oscar Aramburu Bodas; Jordi Grau Amorós; Joan Carles Trullás Vila; Manuel Montero Pérez-Barquero; Gerard Torres Cortada; José Manuel Varela Aguilar; Gonzalo Martínez De las Cuevas; Manuel Méndez Bailón; Nuria Ribas Pizá; Fernando Salgado Ordóñez; Francesc Formiga; Antoni Castro Salomó.