Chemotherapy-associated cardiotoxicity is a common adverse event. Immune checkpoint inhibitors (ICI) – a new class of monoclonal antibodies – have revolutionized the management of various diseases. Their use is expected to increase in the near future and their cardiac side effects have been increasingly recognized.
Clinical caseWe describe a case of a 67-year-old female patient with urothelial carcinoma undergoing treatment with pembrolizumab who presented to the emergency department with progressive fatigue, retrosternal pain and palpitations for three days. On admission she was diagnosed with acute heart failure (HF). The electrocardiogram revealed a right bundle branch block and ventricular bigeminy. Blood tests showed elevated troponin I, while transthoracic echocardiography revealed severe left ventricular dysfunction. Coronary angiography excluded coronary artery disease. Cardiac magnetic resonance revealed moderate left ventricular dysfunction and late gadolinium enhancement typical of myocarditis. Endomyocardial biopsy confirmed the diagnosis of lymphocytic myocarditis. In the first 48h of hospitalization, she developed transient complete AV block. Corticoid and HF therapy were initiated, leading to symptom improvement and disappearance of the rhythm disturbances. She was discharged on the 12th day, maintaining moderate LV dysfunction, which improved only mildly at a subsequent outpatient assessment. She died suddenly 35 days after discharge.
ConclusionLymphocytic myocarditis is a serious cardiac side effect of ICI therapy. Pembrolizumab is increasingly used, so it is important to be aware of its effects, in order to perform an early diagnosis and provide adequate treatment. Corticosteroid therapy seems to be crucial in preventing disease progression and enabling ventricular remodeling.
A cardiotoxicidade associada à quimioterapia é um evento adverso comum. Uma nova classe de anticorpos monoclonais – inibidores dos checkpoints imunes – tem revolucionado o tratamento de várias neoplasias, é expectável num futuro próximo o aumento da sua prescrição. Os efeitos colaterais cardíacos são cada vez mais reconhecidos.
Caso clínicoDescrevemos o caso de uma doente de 67 anos com carcinoma urotelial sob pembrolizumab, admitida no serviço de urgência (SU) por fadiga, dor retrosternal e palpitações com três dias de evolução. No SU foi diagnosticada com insuficiência cardíaca aguda. O ECG relevou bloqueio completo de ramo direito e bigeminismo ventricular e na avaliação analítica com elevação dos biomarcadores de necrose miocárdica.
O ecocardiograma transtorácico revelou disfunção ventricular esquerda grave. A coronariografia excluiu doença coronária. A ressonância cardíaca evidenciou disfunção moderada e padrão sugestivo de miocardite. A biópsia do miocárdio confirmou o diagnóstico de miocardite linfocítica. Nas primeiras 48 h de internamento apresentou períodos de bloqueio AV completo. Foi iniciada corticoterapia e terapêutica neuro-humoral, com rápida melhoria clínica e maior estabilidade elétrica. A doente teve alta ao 12.° dia, mantendo disfunção ventricular moderada. Em ambulatório, foi avaliada apresentando uma ligeira melhoria da fração de ejeção. Ao 35.° dia após alta, a doente faleceu.
ConclusãoA miocardite linfocítica é um evento adverso grave associado à imunoterapia. O uso do pembrolizumab tem aumentado, é importante conhecer os seus efeitos adversos, para permitir um diagnóstico precoce e uma adequada terapêutica. A corticoterapia parece ser crucial na prevenção da progressão da doença e no remodeling ventricular.
Cardiotoxicity is a clinically relevant issue, historically attributed to several cytotoxic agents. It includes multiple cardiac side effects as left ventricular dysfunction and heart failure (HF), myocardial ischemia and infarction, hypertension, QT prolongation and arrhythmias. This toxicity may be reversible/irreversible and can occur soon or several months/years after treatment.1
A new class of antineoplastic agents known as immune checkpoint inhibitors (ICI) has revolutionized the management of a variety of diseases, as melanoma, non-small cell lung cancer, renal cancer or urothelial carcinoma, even in advanced stages.1–5
Nowadays, various patients are eligible for ICI therapy and its use is expected to increase significantly in the near future. There is, therefore, is a need to know its potential adverse effects. A wide spectrum of immune-related adverse events (irAEs) has been described, such as dermatitis, endocrinopathies, colitis, hepatitis, pneumonitis and, largely underestimated, cardiotoxicity.1–4
A number of reported cases of cardiotoxicity have been described in patients treated with isolated ICI or more frequently with combination therapy (more common with nivolumab (anti-programmed death-1 – PD-1) plus ipilimumab (anti-cytotoxic T-lymphocyte-associated antigen 4 – CTLA-4)).1–6
Cardiologists should be vigilant about ICI cardiotoxicity, since it often has an early onset, nonspecific symptoms and fulminant progression.4
Case presentationWe report the case of a 67-year-old female patient with arterial hypertension and a recent diagnosis of non-metastatic urothelial carcinoma who started treatment with pembrolizumab (2 mg/kg every three weeks). Transthoracic echocardiography (TTE) prior to immunotherapy was normal. After five cycles of pembrolizumab, she was admitted to the emergency department with progressive fatigue, episodes of retrosternal chest pain and palpitations for three days.
On physical examination, cardiac auscultation revealed arrhythmic heart sounds and pulmonary auscultation showed bilateral crepitant rales.
A 12-lead electrocardiogram (ECG) showed right bundle branch block and ventricular bigeminy. Analytical study evidenced high levels of troponin I (22 μg/L – normal value <0.4 μg/L) and B-type natriuretic peptide (1801 pg/ml; normal value <100 pg/ml).
The transthoracic echocardiogram revealed severe biventricular dysfunction, with left ventricular ejection fraction (LVEF) of 30% and akinesia of the mid-apical segments of the anterior, inferior, lateral walls. Emergent coronary angiography excluded significant coronary artery disease. The patient was then diagnosed with acute HF and received guideline-recommended HF therapy.7 A reduced dose of betablocker (bisoprolol 2.5 mg) was started to control ventricular dysrhythmia (frequent ectopic beats, with periods of bigeminy). Amiodarone was added later due to maintained electrical instability in the setting of compromised LV function. Both these therapies had to be suspended after 24 hours, due to asymptomatic periods of complete atrioventricular block.
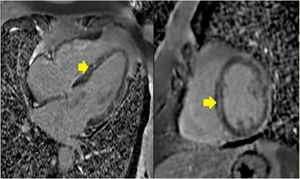
Cardiac magnetic resonance imaging (MRI) performed on day four of hospitalization revealed a dilated left ventricle with moderate systolic dysfunction (LVEF 36%), mid-wall late enhancement involving the middle segments of the ventricular septum and a less evident subepicardial late enhancement involving the middle segments of the lateral wall (video I). There were no focal areas of hyperintensity on T2 weighted imaging to suggest focal myocardial edema at that time, but diffuse inflammation could not be excluded. Myocardial mapping techniques were not yet available at the scanner at this time. Overall, the MRI results were found to be consistent with the clinical diagnosis of myocarditis (Figure 1).
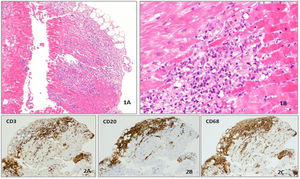
Given the unclear acute biventricular dysfunction under immunotherapy, an endomyocardial biopsy of the ventricular septum was performed (considering the area of late enhancement). Histological examination showed a lymphocytic infiltration of the myocardium with predominance of CD3+ and CD20+ and macrophage infiltrates, confirming the diagnosis of lymphocytic myocarditis (Figure 2).
Histological analysis of endomyocardial biopsy. (1A and 1B) Multifocal inflammatory infiltrate in myocardium, with myocyte dropout (100× and 200× hematoxylin and eosin staining). (2A, 2B and 2C) The inflammatory infiltrate is polyclonal, mostly composed of lymphocytes (CD3 and CD20 positive) and macrophages (CD68 positive) (immunohistochemical analysis).
Due to the suspicion of cardiotoxicity secondary to pembrolizumab, oral prednisolone 2 mg/kg was initiated. HF symptoms rapidly improved and troponin I levels decreased significantly (3 μg/L). The patient was discharged on the 12th day in New York Heart Association class II, with moderate ventricular dysfunction reassessed by TTE (LVEF of 35%). She was referred to the HF outpatient clinic with ramipril (5 mg/day), bisoprolol (1.25 mg/day), furosemide (40 mg/day), spironolactone (25 mg/day) and prednisolone (120 mg/day).
After discharge, the patient remained stable for more than a month, with no clinical worsening. Due to hypotension, ramipril and bisoprolol were not titrated. TTE was repeated 15 and 21 days after discharge, showing mild improvement of LVEF (40%). The patient did not return to immunotherapy. Thirty-five days after discharge, patient had sudden cardiac death, at home. An autopsy was not performed, because the family refused.
DiscussionThe new therapy with ICI has produced frequent and durable antitumor responses in patients with advanced cancers. Nevertheless, immune-related adverse events are frequent and require discontinuation of immunotherapy in nearly 40% of patients, particularly with combination immunotherapy, for example: two cases of myocarditis with complete heart block and myositis after receiving first dose of nivolumab and ipilimumab4; pneumonitis complications and autoimmune hepatitis were also described and required discontinuation of immunotherapy, respectively nivolumab and ipilimumab.3
Myocarditis has rarely been reported in early clinical trials. However, cardiac monitoring is not routinely performed in most immunotherapy trials, so the true incidence is unknown.4
In the literature, there are a few cases of ICI cardiotoxicity reported.2,4,5 A recent multicenter registry from 2013 to 2017 reported a total of 35 patients with ICI-associated myocarditis (prevalence: 1.14%) and about 1/3 secondary to pembrolizumab.6 There is a variation in the reported rates of myocarditis with ICI therapies, however there are relatively common points regardless of the ICI used. According to this registry, myocarditis tends to have an early onset (the median time from starting ICI to clinical myocarditis was 34 days; after 2–3 cycles); 94% of cases had troponin elevation and 89% of cases had an abnormal ECG. In this registry, only 11 patients underwent cardiac biopsy or autopsy in which a T-cell–predominant lymphocytic infiltrate within the myocardium was found, as in our case.6
Predictive biomarkers that would help identify patients at risk for immune-related toxicity are lacking.2 Specifically, all patients who developed cardiotoxicity related to ICI had a baseline echocardiogram with a normal LVEF, as did our patient, and most had a normal ECG. However, troponin levels can be a useful monitoring measurement in these patients. In most who developed cardiotoxicity related to ICI, troponin levels were high at presentation, and a final discharge troponin T ≥1.5 ng/mL was associated with a four-fold increased risk of MACE.6 Therefore, checking troponin levels at baseline and at each cycle may be of value, with immediate referral to cardiology/cardio-oncology for further evaluation if there is an abnormal measurement.
The gold standard for the diagnosis of myocarditis is endomyocardial biopsy.3 Histological analysis of autoimmune lesions after ICI treatment usually shows lymphocytic (CD3; CD8; CD-20 T cell) and macrophage (CD-68) infiltrations, as was shown in our patient's biopsy.3,6
According to the literature, lymphocytic cardiotoxicity associated to ICI resolves with high glucocorticoid doses (1–2 mg/kg/day prednisolone).2 In a retrospective analysis, the majority of patients received steroids and lower steroid doses were associated with higher residual troponin and higher major adverse cardiac events rates.6 In our patient, we started 120 mg prednisolone (2 mg/kg/d) after 24 h of diagnosis in addition to guideline-recommended HF therapy. Her HF symptoms improved, but there was no significant improvement in left ventricular function until her death. A longer period of corticosteroid treatment may be required and there are a few patients reported in the literature who died despite starting corticosteroids while others, who did not receive steroids, suffered permanent decreases in LVEF.2–4 This is compatible once again with a striking individual variability not only in the development of cardiac side effects in patients exposed to this class of antineoplastic agents, but also with regard to their capacity to recover.
Our patient probably died suddenly in the context of a ventricular dysrhythmia or bradyarrhythmia, taking into account the moderate left ventricular dysfunction. However, other causes cannot be ruled out and an autopsy was not performed. It is controversial whether our patient should have been discharged with an implantable cardioverter defibrillator (ICD). In all reported cases, only one patient was discharged with a life vest. According to European guidelines regarding prevention of sudden cardiac death, during the acute phase of myocarditis, ICD implantation should be deferred until resolution of the acute episode.8 As left ventricular function may improve in inflammatory cardiomyopathy due to the natural course of the disease with appropriate HF therapy, implantation of an ICD should not be indicated prematurely. Under European HF guidelines, at least three months of optimized therapy before primary prevention ICD can be offered.7
ConclusionWe report a fatal case of lymphocytic myocarditis secondary to immunotherapy with pembrolizumab, although high-dose corticosteroids were administered. ICI use is growing, so it is important to be aware of the cardiac side effects to enable an early diagnosis and adequate treatment. Predictive biomarkers to identify patients that are most likely at risk for severe ICI cardiotoxicity are lacking. However, troponin levels at baseline and at each cycle may be of value, with immediate referral to cardiology/cardio-oncology for further evaluation if there is an abnormal measurement. In most reported cases of ICI cardiotoxicity, corticosteroid therapy was crucial in preventing disease progression and enabling ventricular remodeling.
Conflicts of interestThe authors have no conflicts of interest to declare.