Heart failure is a disease with high direct and indirect costs. Current treatment includes drugs that alter disease progression and drugs that to improve symptoms. Loop diuretics are the cornerstone of congestion relief for acute management, as well as for chronic stabilization. In heart failure patients, maximal diuretic response is reduced by many individual factors. Diuretic resistance is defined as failure to achieve effective congestion relief despite appropriate or escalating diuretic doses. Its causes include impaired delivery of the diuretic to its luminal site of action, neurohormonal activation, tubular compensatory adaptation and drug interactions. Several strategies can be employed to aid decongestion of patients with impaired diuretic response. These include salt restriction, a higher effective single dose or higher dose frequency of loop diuretics, continuous infusion of diuretics and/or sequential nephron blockade through a synergistic combination of two or more diuretics from different classes. Ultrafiltration has also been found to be another effective and safe therapeutic option and should be considered in patients with refractory diuretic resistance. Overall, there is a lack of high-quality clinical data to guide the choice of treatment strategy and therapy should be tailored on a case-by-case basis.
A insuficiência cardíaca é uma doença com custos diretos e indiretos elevados. A terapêutica atual inclui fármacos que alteram a progressão da doença e fármacos que melhoram a sintomatologia. Os diuréticos de ansa constituem a pedra basilar no alívio da congestão quer na abordagem aguda quer na estabilização crónica. Nos doentes com insuficiência cardíaca, a resposta diurética máxima encontra-se diminuída devido a múltiplos fatores. A resistência aos diuréticos é definida como a ausência de alívio eficaz da congestão apesar de doses apropriadas ou crescentes de diuréticos. As causas de resistência aos diuréticos incluem o compromisso da entrega de diurético no seu local de ação, a ativação neuro-hormonal, a adaptação compensatória tubular e interações medicamentosas. Podem ser implementadas várias estratégias para diminuir a congestão em doentes com resposta diurética insuficiente. Essas estratégias incluem restrição salina, aumento da dose ou frequência dos diuréticos de ansa, infusão contínua de diuréticos e/ou bloqueio sequencial do nefrónio através da combinação de dois ou mais diuréticos de diferentes classes e com efeitos sinérgicos. A ultrafiltração tem-se revelado uma outra estratégia segura e eficaz e deve ser considerada em doentes com resistência aos diuréticos refratária. Verifica-se globalmente uma escassez de dados clínicos de elevada qualidade para guiar a escolha da estratégia terapêutica pelo que a abordagem deve ser adequada caso a caso.
Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure
B-type natriuretic peptide
Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure
Cardiorenal Rescue Study in Acute Decompensated Heart Failure
Chronic kidney disease
Continuous Ultrafiltration for Congestive Heart Failure
Diuretic Optimization Strategies Evaluation
Dopamine in Acute Decompensated Heart Failure II
Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in Acute Heart Failure
Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure
Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure
Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan
Epidemiologia da Insuficiência Cardiaca e Aprendizagem
Heart failure
N-terminal pro–BNP
Nonsteroidal anti-inflammatory drug
Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function
Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy
Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure
Renal Optimization Strategies Evaluation
Renin-angiotensin-aldosterone
Reverse Worsening Renal Function in Decompensated Heart Failure
Ultrafiltration
Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure
Ultrafiltration vs. Diuretics in Decompensated Heart Failure
The incidence of heart failure (HF) is 1% among American patients over 65 years of age.1,2 Portuguese figures from the 2002 Epidemiologia da Insuficiência Cardiaca e Aprendizagem (EPICA) study concluded that the overall prevalence of HF is 4.4%, peaking at 16% in those over 80 years of age.3 It continues to be the primary discharge diagnosis among elderly American patients.4 Hospitalization for HF constitutes an ominous sign, with half of patients readmitted in the subsequent six months and a mortality rate of 25-35% at the end of the first year.4,5 Consequently, HF is a high burden disease with elevated direct and indirect costs.1
Current treatment includes drugs that alter disease progression such as angiotensin converting enzyme inhibitors, angiotensin II receptor blockers or, more recently, sacubitril/valsartan, beta-blockers and mineralocorticoid receptor antagonists – in HF with reduced ejection fraction – and drugs used to improve symptoms such as diuretics, namely loop diuretics.6–8 Loop diuretics are the cornerstone of congestion relief and are widely used for acute management (up to 90% of patients) as well as for chronic stabilization.2,5,8–10
Despite the fact that diuretics themselves are not linked to increased survival,6,8 diuretic efficacy has been shown to prolong event-free survival, regardless of glomerular filtration rate.5,11–13
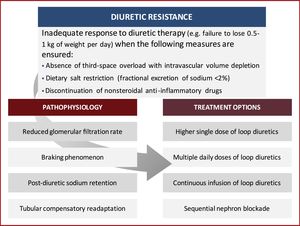
In this review, we discuss the underlying pathophysiology of diuretic resistance in HF patients, while providing several currently available evidence-based pharmacological and non-pharmacological strategies to overcome this problem (Figure 1). The aim of this article is to summarize the existing data and highlight recent research on the subject, providing an up-to-date and practical approach to diuretic resistance.
Diuretic resistance – defining the problemThere is ample variation in the definition of diuretic-resistant patients.13 The general definition refers to the failure to achieve effective congestion relief despite appropriate or escalating doses of diuretics.14,15
Some early reports estimated the prevalence of diuretic resistance to be 20-30% among HF patients.16,17 However, the lack of a formal definition makes it impossible to properly assess the numbers.9,13,15
Recent data link furosemide-equivalent doses of loop diuretics (for intravenous diuretics, 1 mg of bumetanide, 20 mg of torsemide and 40 mg of furosemide11) to changes in several parameters such as weight loss, urine output or natriuresis as means to diagnose diuretic resistance.13 These studies are summarized in Table 1.
Relationship between diuretic efficacy and clinical outcomes in heart failure.
| Author (year) | Metric | Findings in patients with low diuretic efficacy |
|---|---|---|
| Testani et al. (2014)11 | Net fluid loss | Higher all-cause mortality after 5 years (Penn Cohort)/180 days (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Cohort) |
| Valente et al. (2014)5 | Weight loss | Higher heart failure readmissions after 60 daysHigher death, heart failure or renal-related readmissions after 60 daysHigher all-cause mortality after 180 days |
| Voors et al. (2014)22 | Weight loss | Higher death, heart failure or renal-related readmissions after 60 daysNeutral effect on all-cause mortality after 180 days |
| Singh et al. (2014)12 | Urinary sodiumFurosemide concentration | Higher death, transplantation or heart failure readmission after 5 months |
| ter Maaten et al. (2015)18 | Weight lossUrine output | Higher death or heart failure readmission after 30 days |
| Verbrugge et al. (2015)10 | Natriuresis | Higher death or heart failure readmission after 188 days |
| Kumar et al. (2015)19 | Fractional sodium excretion | Higher all-cause mortality after 30 days |
| Ter Maaten et al. (2016)20 | Chloride levels | Higher mortality through 180 days |
| Aronson et al. (2016)56 | Net fluid lossUrine output | Higher all-cause mortality after 6 months |
Adapted from Verbrugge FH, Mullens W & Tang WH (2016).13
Depending on the study, poor diuretic response may be defined as: a weight change of 0 to 2.7 kg per 40 mg of furosemide (or equivalent)18; a urinary diuretic response <1400 ml per 40 mg of furosemide (or equivalent)18; a fractional excretion of sodium at baseline <0.2%19; a urinary sodium concentration and urinary furosemide concentration ratio (both obtained from spot urine samples) <2 mmol/mg12; and/or lower chloride levels at baseline (97 to 103 mEq/l).20 However, the correlation between different metrics remains poor and there is no cut-off to establish actual diuretic resistance.13 Prospective trials are needed to properly validate these metrics.21
Predictors of diuretic resistance, on the other hand, are more firmly established between studies and include: low systemic blood pressure, elevated blood urea nitrogen, HF of ischemic origin and diabetes.5,11,18,22 These studies also found that diuretic resistance is an independent predictor of worse in-hospital outcomes for HF, early post-discharge mortality and increased HF rehospitalization.
Pharmacokinetics & pharmacodynamics of loop diureticsThe relationship between loop diuretic concentration and natriuresis can be illustrated through an S-shaped dose-response curve,14 which means that a minimal concentration must be reached at the site of action before any response is noted.8 A normal dose-response relationship can be distorted by a variety of clinical conditions. In HF patients, the curve is shifted downwards and to the right, which translates into a decreased maximal diuretic response.8 Furthermore, during hospitalization, diuretic response is affected by many individual factors14 including, but not limited to, renal failure, which also shifts the curve to the right, meaning a higher dose of diuretics is needed to achieve the same degree of natriuresis.8
Pathophysiology of diuretic resistanceThe pathophysiology of diuretic resistance is complex and several causes may be involved (Table 2).15 It stems from multiple factors, including reduced delivery of the diuretic to its luminal site of action, neurohormonal activation, tubular compensatory adaptation and drug interactions.15
Causes of diuretic resistance.
| Incorrect diagnosis |
| Venous edema |
| Lymphatic edema |
| Third space overload with intravascular volume depletion |
| Nonadherence to recommended sodium and/or fluid restriction |
| Poor diuretic delivery to the nephron lumen |
| Nonadherence |
| Dose too low or too infrequent |
| Poor absorption (example: edematous gut) |
| Hypoalbuminemia and nephrotic syndrome |
| Hepatic cirrhosis |
| Reduced diuretic secretion |
| Tubular uptake of diuretic impaired by uremic toxins |
| Decreased kidney blood flow |
| Decreased functional kidney mass |
| Insufficient kidney response to drug |
| Low glomerular filtration rate |
| Decreased effective intravascular volume despite elevated total extracellular fluid volume |
| Activation of the renin-angiotensin-aldosterone axis and renal sympathetic nerves |
| Increased sodium delivery and absorption in distal tubular segments |
| Compensatory retention of sodium after the effective period of the diuretic |
| Nephron adaptation (hypertrophy and hyperplasia of distal tubular cells) |
| Use of nonsteroidal anti-inflammatory drugs |
Adapted from Hoorn EJ & Ellison DH (2017).36
Reduced delivery of the diuretic to its site of action is closely related to its decreased bioavailability.
In HF patients, increased peripheral and bowel wall edema leads to reduced absorption of the diuretic, with a more marked effect when oral furosemide is used.21
HF itself as well as concurrent chronic kidney disease (CKD) (urate and other competing organic acids) may lead to decreased glomerular filtration rate, which in turns leads to impaired secretion of diuretics (namely furosemide) by the organic acid transporter into the proximal tubule. Reduced glomerular filtration rate can, therefore, reduce delivery or reduce active secretion of loop diuretics into their site of action.23 Moreover, CKD has been proposed as a contributing factor to the development of HF overall, regardless of left ventricular ejection fraction. CKD leads to volume retention, altered calcium–phosphate metabolism, hyperparathyroidism, vitamin D deficiency, anemia, and the accumulation of uremic toxins.24 Renal dysfunction caused by intra-abdominal hypertension and cardiorenal syndrome are also plausible mechanisms of diuretic resistance through venous congestion.9,25 Intra-abdominal hypertension relief improves renal perfusion, renal filtration and diuresis. It is usually present in up to 60% of acutely decompensated HF patients.25 It is very important to emphasize the need to detect third-space overload as opposed to intravascular overload because both the kidneys and diuretic therapy can only act in vascular overload. Persistent diuretic use in patients who are already suffering from intravascular volume depletion further activates the renin-angiotensin-aldosterone (RAA) axis and makes diuretic resistance dependent on renal blood flow. Urinary sodium and chloride measurements may indicate when vascular volume has been optimized because they decrease as euvolemia approaches.13 These may serve as more reliable markers of decongestion as opposed to the clinical signs and symptoms traditionally used to guide decongestive therapy.26 Clinical signs and symptoms lack sensitivity and specificity but do raise the need for further clinical evaluation. Natriuretic peptides are helpful for diagnosis and prognosis but lack the power to properly monitor volume status.26 Newer approaches point to quantitative blood volume analysis as means to differentiate hypervolemia profiles. Appropriate profiling of volume overload in HF, according to blood volume, has therapeutic implications and may aid patients with diuretic resistance, redirecting them to other forms of decongestion.26
Another mechanism of diuretic activity impairment involves increased re-absorption of sodium and chloride in the proximal tubule, leading to decreased delivery of these substrates to the distal areas of the nephron where loop diuretics act. This mechanism causes diuretic resistance through decreased substrate availability to the sodium-potassium-chloride cotransport system.13
Albumin levels also correlate to diuretic action because they are high affinity albumin-binding molecules (>90%).9 Hypoalbuminemia increases the drug distribution volume and prevents suitable kidney delivery. On the other hand, high levels of albuminuria decrease loop diuretic delivery. Increased urine albumin binds to diuretics, preventing their ligation to the sodium-potassium-chloride receptors and thus impairing their action.9
Neurohormonal activationNeurohormonal activation is strongly related to RAA axis upregulation. Loop diuretics can activate the RAA axis through a variety of mechanisms.15 They induce renin secretion through the direct blockade of the macula densa sodium-potassium-chloride cotransport system, thus leading to increased renin and aldosterone in a volume-independent pathway.8 Furthermore, diuretics induce renal prostacyclin production, which increases renin secretion. Finally, diuretics induce volume contraction, thus activating renin secretion through vascular stimulation.8 RAA axis activation eventually leads to increased sodium reabsorption, prompting the onset of post-diuretic sodium retention and the braking phenomenon.15 Post-diuretic sodium retention is one of the processes through which diuretic resistance may be established and it arises as soon as the concentration of diuretic in the tubular fluid drops below the therapeutic threshold.15 A negative net sodium balance in the 24 hours between natriuresis and post-diuretic sodium retention may not be achieved in the event of dietary noncompliance, rendering the diuretic effect insignificant.15 The braking phenomenon, on the other hand, is defined as the decrease in diuresis volume after multiple same-dose administrations of diuretic. This is linked to RAA axis activation and compensatory changes in the nephron.9
Tubular compensatory readaptationTubular readaptation is another mechanism that helps explain reduced diuretic response.21 Owing to the abovementioned activation of the RAA axis, as well as the braking phenomenon, proximal tubular reabsorption arises, leading to increased sodium uptake in this area of the nephron.21 Simultaneously, the chronic use of loop diuretics – which inhibit sodium uptake in the loop of Henle – leads to increased sodium delivery to the distal tubular system, resulting in compensatory hyperplasia and hypertrophy.9 This means that the patient would retain more sodium and thus water than a diuretic-naïve patient.13,21 This resistance mechanism can be overcome using a sequential nephron blockade with thiazide diuretics.9
Drug interactionsSome drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs), can reduce the effect of diuretics.21
NSAIDs may cause diuretic resistance in a number of ways, particularly: decreased prostaglandin synthesis, decreased renal vasodilation, increased renal reabsorption in areas of the nephron other than the loop of Henle and hypertension.9 Evidence regarding the effect of low-dose aspirin (<1 mg/kg/day) on diuretic response in particular is more scarce and controversial. A previous study reported that chronic low-dose aspirin could profoundly affect platelet prostaglandin production without affecting diuretic-stimulated renal prostacyclin production or plasma renin activity.27 However, more recently, Jhund et al. demonstrated that the venodilation that occurs following furosemide administration could be inhibited by both high and low dose-aspirin.28 Furthermore, Hall noted an important reduction in the need for diuretics when daily aspirin administration was stopped.29 There is also some evidence that aspirin, even at a low dose, may neutralize the favorable effects of angiotensin-converting enzyme inhibitors by blocking prostaglandin production and enhancing the vasoconstrictor potential of endothelin.30 In patients with HF, aspirin should be avoided wherever possible and other antithrombotic agents that respect the integrity of prostaglandin metabolism should be considered.
Treatment options for diuretic resistanceOverall, there is a lack of high-quality clinical data to guide the choice of treatment strategy to overcome diuretic resistance.31 Several strategies can be employed to aid decongestion in patients with acute HF manifesting an impaired diuretic response. These include diuretic and nondiuretic strategies (Table 3).21
Strategies for treating diuretic resistance.
| Strategy | Summary of evidence | Recommendation |
|---|---|---|
| Initial measures | ||
| Intravenous diuretics | Improved pharmacokinetics in compensated heart failure | Initial measure in hospitalized patients |
| Increase diuretic dose | Doses of ≥500 mg/day of oral furosemide were safeHigh intravenous doses were safe and were more effective | Consider in mildly symptomatic ambulatory patients |
| Use alternative loop diuretic (bumetanide and torsemide) | Greater enteral absorption and less affected by edematous states | Consider in case of poor response to oral furosemide |
| Continuous infusion | Lower total daily doses to elicit the same degree of natriuresis compared to bolus doses without better symptom relief or creatinine improvements | Consider in case of inadequate response to bolus doses |
| Combination of intravenous loop diuretics with one or more diuretics from different classes | - Thiazide/Metolazone – Increased urine sodium and/or weight loss- Acetazolamide – increased diuresis | Consider in case of inadequate response to increasing doses of IV loop diuretics |
| - Mineralocorticoid receptor antagonist – increased diuresis and faster symptom relief | Consider in ambulatory or outpatients with an inadequate response to loop diuretics | |
| Advanced measures | ||
| Hypertonic saline infusion | Improved diuresis and renal function, and shortened hospitalizations | Consider when the above options have failed |
| Dopamine | Similar urine output when added to low-dose intravenous furosemide compared with high-dose intravenous furosemide aloneSubsequent trials have failed to demonstrate a benefit | Consider only when all other options have failed |
| Nesiritide | No improvement in urine output, hospital readmission or mortality | Should not be used in loop diuretic resistance |
Adapted from Bowman, Nawarskas & Anderson (2016).31
Before considering the following treatment options, other causes of apparent diuretic resistance, such as third-space overload with intravascular volume depletion, must be ruled out.
Salt restrictionDietary sodium restriction is a key determinant of diuretic efficacy. When dietary sodium intake is high, post-diuretic sodium retention compensates almost entirely for the loop-diuretic-induced sodium loss. Conversely, if sodium intake is restricted, post-diuretic sodium retention is minimized, resulting in a negative fluid and sodium balance.32 Thus, restricting sodium intake to less than 100 mEq/day mitigates the effect of post-diuretic sodium retention and helps achieve a negative sodium balance. A 24-hour urinary sodium excretion of more than 100 mEq/day or a fractional excretion of sodium value >2% indicates non-compliance with sodium restriction and rules out true diuretic resistance.15
Discontinue concomitant use of nonsteroidal anti-inflammatory drugsConcomitant use of NSAIDs is a major cause of diuretic failure and discontinuing them can significantly improve diuretic effectiveness.15
Establish the effective single doseDiuretics have a dose-response curve and the effect only begins once the diuretic level reaches a therapeutic threshold within the renal tubular lumen. In conditions such as CKD and cardiorenal syndrome, the dose-response curve shifts downwards and towards the right. This means that these patients need higher doses of loop diuretics to achieve the therapeutic drug level at the site of action. Diuretic doses below said threshold are ineffective, so a higher effective single loop diuretic dose is needed rather than administering an inadequate dose more frequently.15
Increase dose frequency of loop diureticsBecause most loop diuretics are short acting, increasing the dose frequency can help overcome post-diuretic sodium retention and restore diuretic response.15
Diuretic substitutionGastrointestinal absorption and the bioavailability of different diuretics belonging to the same class can vary considerably and this could be a factor behind a poor response. Furosemide has a bioavailability of about 50%, whereas torsemide and bumetanide have almost complete absorption (80-100%). At times, replacing furosemide with comparable doses of bumetanide or torsemide can be enough to improve diuresis.15
Intravenous diureticsSometimes, administering diuretics intravenously instead of orally is all that is needed to improve diuresis. Oral absorption may be altered in the presence of gastrointestinal edema, gastroparesis and delayed gastric emptying. Drug concentration at the site of diuretic action in the tubule lumen may be inadequate, due to decompensated HF, renal hypoperfusion or impaired secretion as a result of hypoalbuminemia.32
Compared to bolus doses, continuous diuretic infusion may be more effective in improving diuresis. It may decrease fluctuations in intravascular volume, resulting in a more gradual and relatively constant hourly urine output and limiting the effect of post-diuretic sodium retention. Some studies found that furosemide administered as a continuous infusion was more effective than intermittent bolus doses, since significantly less furosemide was required to produce the same diuresis and due to the elimination of a diuretic-free interval (during which compensatory sodium retention occurs). Said studies found no significant differences in adverse effects and no change in serum creatinine or hospital mortality.33,34 Other studies reported that both regimens were equally effective in achieving a negative fluid balance.32 In the Diuretic Optimization Strategies Evaluation (DOSE) trial, there were no significant differences in patients’ global symptom assessment or in the change in renal function between the two strategies.2 Despite the conflicting evidence, pharmacodynamic concepts support the improved efficacy of continuous infusion of all loop diuretics except ethacrynic acid. A bolus dose of a loop diuretic should be administered before initiating a continuous infusion or when the infusion rate is increased in order to decrease the time for the drug's onset of action (Table 4).32,35
Dosage regimens for continuous intravenous diuretic administration.
| Creatinine clearance (ml/min) | Loading dose (mg) | Infusion rate (mg/h) | ||
|---|---|---|---|---|
| All levels | <25 | 25-75 | >75 | |
| Furosemide | 40 | 20 then 40 | 10 then 20 | 10 |
| Bumetanide | 1 | 1 then 2 | 0.5 then 1 | 0.5 |
| Torsemide | 20 | 10 then 20 | 5 then 10 | 5 |
Adapted from Brater (2011).35
A sequential sodium uptake blockade in different nephron segments by means of a combination of two or more diuretics from different classes may produce an additive or synergistic mechanism of action and diuretic response, and can be an effective approach in resistant cases (Table 5).15 For edematous disorders other than liver cirrhosis and ascites, the evidence for specific diuretic combinations is less clear.36 The use of a loop diuretic and a thiazide or thiazide-like diuretic with or without a potassium-sparing agent is most common in practice.15 Nevertheless, in the absence of evidence on the comparative efficacies of the various diuretic combinations, choosing a strategy should be based on patient-specific factors and the side effect profiles of the different combinations.31
Combination diuretic therapy.
| To an effective or maximal safe dose of a loop diuretic add: |
| Distal convoluted tubule diuretics |
| Metolazone 2.5-10 mg per os daily (duration or frequency adjusted based on the target weight) |
| Hydrochlorothiazide (or equivalent) 25-100 mg per os daily |
| Chlorothiazide 500-1000 mg intravenously |
| Proximal tubule diuretics |
| Acetazolamide 250-375 mg daily or up to 500 mg intravenously |
| Potassium-sparing diuretics |
| Spironolactone 100-200 mg daily |
| Amiloride 5-10 mg daily |
Adapted from Ellison (2001).8
Thiazide diuretics inhibit sodium reabsorption in the distal convoluted tubule and can thus counteract compensatory distal tubular hypertrophy.31 Metolazone and hydrochlorothiazide are the two thiazides most commonly used in combination with furosemide, although there is no clear evidence that one is superior to the other, neither in terms of their efficacy in increasing diuresis nor their safety with regard to renal function and electrolyte abnormalities.32,37,38 When initiating combination therapy, thiazides should be administered before intravenous loop diuretics to allow enough time for the full blockade of the distal nephron.32 Case studies and small observational trials reported effective diuresis in 75-90% of patients who received thiazide diuretics in addition to loop diuretic therapy.31 However, one drawback of thiazide-type diuretics is that they limit the kidneys’ capacity to produce diluted urine and thus free water clearance and they should therefore be avoided in hypotonic hyponatremia.13
There is a paucity of data regarding specific mineralocorticoid receptor antagonists use in acute HF and the combination of spironolactone and loop diuretics has not been shown to be synergistic. Nevertheless, said drugs exhibit mild but effective natriuretic effects and minimize potassium wasting by loop diuretics.8,13 Moreover, spironolactone or eplerenone are recommended in all symptomatic patients (despite treatment with an angiotensin-converting enzyme inhibitor and a beta-blocker) with HF and a reduced ejection fraction in order to reduce mortality and HF hospitalization.31 Therefore, there may be a strong rationale to continue and even increase dosing of these drugs when the glomerular filtration rate is stable and serum potassium levels are less than 5.5 mEq/l.8,13
While the diuretic and natriuretic capacity of acetazolamide is poor on its own, it could well be a very efficient booster of diuretic efficacy. The combination of acetazolamide and a loop diuretic can be very effective, blocking more than 90% of sodium reabsorption in the nephron. Moreover, it reduces renin release with potentially favorable effects on neurohormonal activation. However, there are currently no data on the benefits of acetazolamide as add-on therapy and long-term use can cause metabolic acidosis.13,15
Combination therapy is associated with a significant increase in adverse effects such as electrolyte imbalances, dehydration and renal impairment. It requires careful monitoring and is best reserved for the occasional patient with high resistance to loop diuretics.15,32
Management of intra-abdominal pressureIntra-abdominal hypertension is defined as a sustained intra-abdominal pressure of 12 mmHg or above. Splanchnic and interstitial congestion may cause elevated intra-abdominal pressure in the absence of ascites in acute decompensated HF. In such patients, a rise in intra-abdominal pressure increases renal venous pressure, thereby reducing the transrenal perfusion gradient and renal perfusion. Elevated intra-abdominal pressure also causes increased renal interstitial pressure that opposes net filtration pressure. Both contribute to renal impairment and diuretic resistance. When intravenous loop diuretic therapy fails, measuring intra-abdominal pressure is an inexpensive and minimally invasive procedure that rules out a diuretic resistance cause. If intra-abdominal hypertension or abdominal compartment syndrome (defined as a sustained intra-abdominal pressure of >20 mmHg which is associated with new organ dysfunction) is identified, a reduction in intra-abdominal pressure by mobilizing third-space fluid can be achieved through a combination of diuretics, vasodilators and/or inotropes. Abundant ascites can be managed with paracentesis, ultrasound or computer tomography guidance if needed. In certain patients, ultrafiltration (UF) may be appropriate. The therapeutic aim is to achieve an abdominal perfusion pressure (calculated as the mean arterial pressure minus the intra-abdominal pressure) of over 60 mmHg (with an intra-abdominal pressure of 5 to 7 mmHg), which signifies a favorable outcome (improvement in renal perfusion, renal filtration and diuresis).25
Infusion with albuminSimultaneous infusion of a diuretic and albumin could slightly improve diuretic resistance. A meta-analysis of eight randomized clinical trials of adults with hypoalbuminemia, comparing the co-administration of loop diuretics and albumin versus loop diuretics alone, found transient effects of modest clinical significance with the former strategy.39 However, this intervention should only be considered in severely hypoalbuminemic patients when the approaches discussed above have failed.15
Renal-dose dopamineLow doses of dopamine (<3 μg/kg/min) selectively work on peripheral dopaminergic receptors resulting in vasodilation in the renal, coronary, splanchnic and cerebral circulations. Two recent trials of dopamine in acute HF – the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial and the Renal Optimization Strategies Evaluation (ROSE) trial – have shown no added benefit with the addition of dopamine to standard therapy with high-dose diuretics. Thus, on the basis of current data, dopamine has no role in nonhypotensive patients with acute HF. In the absence of cardiogenic shock, however, the role of low-dose dopamine in acute HF with hypotension merits further study.21,31
Alternative pharmacological therapiesHypertonic saline works osmotically to pull free water from the interstitial fluid into the renal vasculature. In addition to increasing renal blood flow, it improves sodium delivery to the loop of Henle, thus restoring some of the loop diuretics’ effect. Several studies have reported better diuresis, improved renal function and shorter hospital stays when hypertonic saline is added to loop diuretic therapy.31
Nesiritide is a synthetic B-type natriuretic peptide (BNP) approved by the Food and Drug Administration for symptomatic relief due to its favorable effects on hemodynamics, dyspnea and renal function. However, both the ROSE trial and ter Maaten et al. (2015) found no additive effect of using low-dose nesiritide added to diuretic therapy in terms of decongestion or improved renal function. Experimental research has shown that renal delivery of BNP had significantly greater beneficial effects than systemic delivery. It could be that a higher systemic dose is needed; however, the usage thereof would increase the incidence of adverse effects such as hypotension.18,40
Furthermore, it is worth noting that HF trials on nesiritide and dopamine have not been specific to patients exhibiting a resistance to loop diuretics.31
Vasopressin-2-receptor antagonists may promote aquaresis by blocking the effects of vasopressin on the vasopressin 2 receptors located in the collecting ducts, thus blocking the re-absorption of free water. This promotes water clearance without affecting sodium balance. In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial, tolvaptan at a dose of 30 mg once daily for a minimum of 60 days had no effect on total mortality or HF hospitalization when compared to placebo.21,41
Adenosine antagonists can potentially increase glomerular filtration and enhance the diuretic effect of diuretic drugs. However, the Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) trial did not report any beneficial effects on congestion or renal function and was associated with an increased risk of seizures.21,42
Glucocorticoids may promote diuresis and protect renal function in patients with acute HF. Liu et al. reported the effects of prednisolone in 13 congestive HF patients with significant volume overload and diuretic resistance who had failed to respond to a conventional sequential nephron blockade treatment strategy. They reported an improvement in diuresis, clinical status and renal function.43 The same author later reviewed the available evidence and concluded that the short-term use of glucocorticoids, when added to maximum conventional therapy, can potentiate renal responsiveness to diuretic therapy in patients with HF.44 However, larger randomized double-blind placebo-controlled studies are warranted to demonstrate their safety and efficacy in such patients. The proposed mechanism of action of glucocorticoids includes increased expression of natriuretic peptide receptor-A in the kidney and the hypothalamus, which appear to be reduced in patients with HF, also increasing renal blood flow through dilatation of the renal vasculature via increased renal prostaglandin, nitric oxide and dopamine production.21,43,44
Levosimendan was studied in patients presenting with acute HF in the Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy (REVIVE) studies. These showed that levosimendan improved renal function and diuretic response in such patients. However, there was also an increased risk of arrhythmia and hypotension.21
Ularitide, a human endogenous natriuretic peptide expressed in the kidney, which induces natriuresis and diuresis by binding to a specific natriuretic peptide receptor, was investigated in patients with acute HF in the Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure (TRUE-AHF) trial. Packer et al. reported favorable physiological effects (greater reductions in systolic blood pressure and in levels of N-terminal pro–BNP [NT-proBNP] than the placebo group, without affecting cardiac troponin levels). However, short-term treatment neither affected the initial 48-hour clinical course nor reduced long-term cardiovascular mortality.21,45
In the Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure (RELAX-AHF) trial, serelaxin, a human recombinant form of the vasodilator relaxin, showed no significant effect on diuretic response, but did have beneficial effects in preventing organ damage in patients with acute HF who were diuretic-resistant.21,46 The Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF (RELAX-AHF-2) trial was designed to confirm serelaxin's effect on these clinical outcomes but it did not meet either of its primary endpoints. There was no difference in cardiovascular mortality at 180 days and the trend for reducing worsening HF through day five with serelaxin was not statistically significant.47
There are several other agents under investigation, which could play a role in aiding decongestion of patients presenting with acute HF, such as omecamtiv mecarbil and TRV027.21
Omecamtiv mecarbil is a selective cardiac myosin activator that increases myocardial function in healthy volunteers and in patients with chronic HF. Its effects on patients with acute HF were evaluated in the Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure (ATOMIC-HF) trial. This study showed that omecamtiv mecarbil may improve dyspnea scores when higher doses were used in comparison to placebo; however, it did not significantly improve overall dyspnea scores – the primary endpoint of the study.48
TRV027 is a novel ligand of the angiotensin II type 1 receptor, selectively antagonizing the negative effects of angiotensin II, while preserving the potential pro-contractility effects of angiotensin II type 1 receptor stimulation. Its safety and efficacy were assessed in the Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure (BLAST-AHF) trial and, although well tolerated, TRV027 did not improve clinical status through 30-day follow-up compared to placebo.49
Further studies are needed to evaluate the safety and efficacy of these drug candidates in a larger group of patients with acute HF.
UltrafiltrationUF is very effective at removing plasma fluid from blood across a semipermeable membrane that allows small molecules to pass through along its pressure gradient to the ultrafiltrate fluid.21
Small studies suggest that UF improves pulmonary and peripheral edema, lung function and hemodynamics without adverse effects on renal function. The fluid removal rate is reevaluated using clinical assessment and serial hematocrit measurements to ensure appropriate vascular compartment refill.21
The recent development of veno-venous peripheral UF has positioned this technique as a potential alternative to loop diuretics in acute HF.21
The Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial, a multicenter randomized controlled trial involving forty patients, found that UF was feasible, well-tolerated, and resulted in significant weight loss and fluid removal.50
Favorable outcomes were also reported in the Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial. This prospective, randomized, multicenter trial involving 200 patients, found that UF safely produces greater weight and fluid loss than intravenous diuretics, reduces 90-day resource utilization for HF and is an effective alternative therapy.51
In the Ultrafiltration vs. Diuretics in Decompensated HF (ULTRADISCO) study, a prospective, randomized, open-label, single-center study which included 30 patients, the use of UF was associated with greater hemodynamic stability and with a greater reduction in plasma levels of NT-proBNP and aldosterone compared to diuretic infusion.52
In the Continuous Ultrafiltration for Congestive Heart Failure (CUORE) trial, UF as a first-line treatment in patients with severe congestive HF was associated with prolonged clinical stabilization and greater freedom from rehospitalization for congestive HF compared to standard medical therapy alone.53
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), however, found a stepped pharmacologic-therapy algorithm to be superior to UF for the preservation of renal function at 96 hours, with a similar amount of weight loss seen with the two approaches and a higher rate of adverse events observed with UF.54
Results of the Reverse Worsening Renal Function in Decompensated Heart Failure (REWORD-HF) study (which ended in April 2017) are still pending and will help determine whether fluid removal by UF is superior to different pharmacological approaches in acutely relieving congestion and preventing further deterioration in renal function and whether it results in longer admission-free survival 90 days after enrolment in patients with decompensated HF and cardiorenal syndrome.
Seven randomized controlled trials, including several of those mentioned above, were submitted for a meta-analysis. UF was found to be an effective and safe therapeutic strategy, resulting in greater weight loss and fluid removal without affecting renal function, mortality or rehospitalization.55
The 2016 European Society of Cardiology guidelines state that there is no evidence favoring UF over loop diuretics as first-line therapy in patients with acute HF. The former should thus be confined to patients who fail to respond to diuretic-based strategies.
ConclusionDiuretic resistance has emerged as an independent factor behind worse HF patient outcomes, namely in-hospital worsening, early post-discharge mortality and rehospitalizations. While several mechanisms help to explain their reduced response to diuretics, the definition of the problem itself remains elusive. More recent evidence is leaning towards the coupling of parameters such as weight loss and urine output to diuretic dose, but several challenges remain. Non-pharmacological measures and a few medical options, such as continuous infusion of diuretics and sequential nephron blockade, may be used to overcome diuretic resistance. Nevertheless, disease progression may warrant more invasive methods for fluid removal. Therapy must be tailored on a case-by-case basis.
Conflicts of interestThe authors have no conflicts of interest to declare.