Cardiovascular disease is the leading cause of death worldwide and ischemic heart disease is the most frequent etiology, with high economic costs for both treatment and diagnosis. Over the past two decades, the assessment of patients with this disease has undergone various changes, with cardiac positron emission tomography (PET) emerging as a powerful and versatile imaging exam for diagnosis and risk stratification of these patients. This review aimed to assess the utility of this exam, particularly through quantification of myocardial blood flow and myocardial flow reserve in the diagnosis and risk stratification of coronary artery disease. Compared to other imaging methods, measurement of these parameters by cardiac PET provides a better characterization of coronary artery disease, with particular value in microvascular and balanced multivessel disease.
A doença cardiovascular é a causa mais comum de morte no mundo e a doença cardíaca isquémica mantém-se como a principal etiologia, com elevados custos económicos tanto no seu tratamento como no seu diagnóstico. Nas últimas duas décadas a avaliação dos doentes com esta patologia tem sofrido várias modificações e a tomografia de emissão de positrões cardíaca tem emergido como um poderoso e versátil exame de imagem que permite diagnosticar e estratificar o risco destes doentes. Esta revisão teve como objetivo avaliar a utilidade deste exame, nomeadamente através da quantificação do fluxo sanguíneo total miocárdico e da reserva de fluxo sanguíneo miocárdico, no diagnóstico e estratificação de risco da doença arterial coronária. Comparativamente com outros métodos de imagem, a aferição por tomografia de emissão de positrões cardíaca destes parâmetros contribui para uma melhor caracterização da doença arterial coronária, com especial relevância na doença microvascular e multivascular equilibrada.
According to the 2016 World Health Organization report, cardiovascular disease, especially coronary artery disease (CAD), is the leading cause of death and disability worldwide. The approach to patients with CAD focuses not only on treatment and prevention, but also on diagnosis, which combines clinical characteristics with diagnostic exams.
Positron emission tomography (PET) was for years used exclusively for cardiological studies and was only available in advanced research institutions. However, recent decades have witnessed enormous technological and biological advances that have led to this powerful tool being increasingly used routinely in contemporary nuclear cardiology laboratories, where it is now a valuable adjunct to daily clinical practice.1 Indications for cardiac PET have widened in parallel,2 mainly because the images obtained with this modality are of high spatial resolution and reproducibility without subjecting patients to high radiation doses. Another advantage of the exam is that it can provide non-invasive quantification of absolute myocardial blood flow (MBF) in ml/min/g of tissue.
This review examines the fundamental principles of cardiac PET imaging, the characteristics of currently available tracers for assessing myocardial perfusion, and methods for quantifying MBF and myocardial flow reserve (MFR). We also set out to compare cardiac PET with other imaging methods, in order to determine the value of MFR assessment in the detection and characterization of CAD, particularly for microvascular and balanced multivessel disease.
MethodsThe first four sections of this paper (technical considerations, radiotracers, quantification of MBF and MFR, and comparisons with other techniques) constitute a narrative review that serves as an introduction to the fifth section, on the advantages of MFR in detecting microvascular and balanced multivessel disease. To this end, we analyzed 82 studies identified by searching the PubMed and EMBASE databases for relevant research articles, systematic reviews and guidelines, with the following inclusion criteria: (i) publication date between January 2000 and July 2018; (ii) English, Portuguese, Spanish or French language; (iii) access to the complete text; (iv) appropriateness to the subject under study; and (v) relevance. Studies that were considered to be irrelevant to the subject under study or to duplicate content found in other studies were excluded.
For the fifth section, only research articles investigating the use of MFR in microvascular and balanced multivessel disease were analyzed, with the following inclusion criteria: (i) publication date between January 2008 and July 2018; (ii) English, Portuguese, Spanish or French language; (iii) studies in humans; and (iv) access to the complete text. The PubMed and EMBASE databases were searched using the following search terms: (“Coronary Artery Disease AND Positron Emission Tomography) AND (Myocardial Blood Flow OR Myocardial Flow Reserve OR Coronary Flow Reserve OR Myocardial Perfusion”). After the initial bibliographic searches (n=266 and n=131), duplicates and literature reviews were excluded and then the title and abstract of each study was analyzed in order to exclude all articles that did not specifically study multivessel or microvascular CAD. The complete texts of all potentially relevant studies were then retrieved and analyzed (n=27). Of these, only seven assessed the utility of MFR quantification in multivessel or microvascular CAD, using statistical criteria (sensitivity, specificity, area under the curve, endpoints and hazard ratios) that could be compared for diagnosis, risk stratification and prognosis, and these were included in this review. The other 20 were case reports or analyzed specific populations such as diabetic or obese patients or compared patients before and after transplantation or revascularization, and were therefore excluded from the analysis.
Technical considerations of positron emission tomography: a brief explanation of image acquisition and interpretationPET imaging is based on the use of radionuclides that decay and emit positrons. The radionuclides, combined into radiotracers, are distributed in tissues of the human body, and when positrons are emitted they collide with nearby electrons, resulting in mutual annihilation that produces energy in the form of two 511-keV photons moving in approximately opposite directions. Since the positron beam is small (a few mm), their decay can be considered to have occurred along the straight line described by the two annihilation photons. PET scanners contain detector rings that convert the energy of the annihilation photons into an electrical signal. The basic principle of PET is thus the detection of photons at the same time arriving at a ring detector from opposite directions (‘coincidence events’). The spatial resolution of PET scanners is currently 4-7 mm.
In clinical practice, PET myocardial perfusion scans are usually classified visually using qualitative criteria. The relative distribution of the radiotracer is assessed at rest and during stress (hyperemia). Myocardial perfusion defects are generally classified according to their extent, severity and location. Current guidelines also recommend a semi-quantitative assessment using a five-point scale in a 17-segment model of the left ventricle.3,4 These scores can be used for resting and stress assessments, and the differences between them can be analyzed to identify reversible defects. Fixed defects are indicative of myocardial scarring, while stress-induced reversible hypoperfusion indicates ischemia.5
In addition to qualitative and semi-quantitative classifications, PET can also provide absolute perfusion quantification, and several software packages are available that can automatically calculate MBF for different myocardial territories.
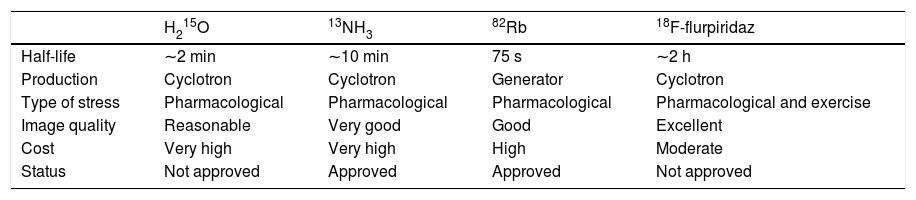
RadiotracersOf the various radiotracers available for perfusion scanning, rubidium-82 (82Rb), 13N-labeled ammonia (13NH3), and O15-labeled water (H215O) are most commonly used in clinical practice6 and have all been validated with high reproducibility.7,8 An emerging perfusion tracer, 18F-flurpiridaz, has considerable potential but is not yet available for clinical use.9,10
The characteristics of each radiotracer, including advantages and disadvantages, are described below and summarized in Table 1. It should be borne in mind that no perfusion tracer has all the ideal characteristics, and so various factors will determine the choice between them, which will be based on practical considerations as well as the purpose of the exam.
Characteristics of radiotracers available for cardiac positron emission tomography.
| H215O | 13NH3 | 82Rb | 18F-flurpiridaz | |
|---|---|---|---|---|
| Half-life | ∼2 min | ∼10 min | 75 s | ∼2 h |
| Production | Cyclotron | Cyclotron | Generator | Cyclotron |
| Type of stress | Pharmacological | Pharmacological | Pharmacological | Pharmacological and exercise |
| Image quality | Reasonable | Very good | Good | Excellent |
| Cost | Very high | Very high | High | Moderate |
| Status | Not approved | Approved | Approved | Not approved |
13NH3: 13N-labeled ammonia; 82Rb: rubidium-82; H215O: O15-labeled water.
H215O is only used in Europe and Asia, since the US Food and Drug Administration (FDA) has yet to approve it for clinical use. This tracer has unique characteristics for determining MBF, being metabolically inert and freely diffusible, and hence presenting a linear relationship between first-pass extraction and perfusion. Other advantages of this radiotracer include its short half-life and low radiation dose, enabling repeated measurements in short time intervals.11 However, this short half-life means that although pharmacological stress testing is feasible, exercise stress testing is not. Other disadvantages in clinical practice include the need for an on-site cyclotron to produce the radionuclide and its poor signal-to-noise ratio, which results in images that are suboptimal for assessment purposes.9 However, recent years have seen the development of digital subtraction techniques and software capable of automatically generating parametric images that can be classified qualitatively using H215O, and these advances may allow the use of this tracer in clinical practice in the future.12–14
13NH3 has been approved by the FDA since 2000 to assess myocardial perfusion in patients with documented or suspected CAD.15,16 This tracer diffuses passively through capillary and interstitial tissue into myocytes, and its relationship with MBF is non-linear due to the roll-off phenomenon. Its first-pass myocardial extraction of about 80% and its rapid clearance from the blood mean that perfusion images of excellent diagnostic quality can be obtained. Another advantage of 13NH3 is its long half-life of around 10 min, which enables its use with exercise stress testing as well as for pharmacological stress.11,17 Its main disadvantage is the need for cyclotron production, and thus although its half-life is significantly longer than that of H215O, it must also be produced by a cyclotron located in the laboratory itself.11
82Rb has been approved by the FDA since 1989 and is widely used in the US, Canada, Japan and Europe.18 It is a potassium analog that is actively taken up by cardiomyocytes via the ATP-dependent sodium/potassium pump. One of its advantages in clinical practice is that it is produced in a generator and thus does not require use of a cyclotron.19 Its half-life is extremely short (around 76 s), making exercise stress testing unfeasible, and it can therefore only be used for pharmacological rest/stress testing.11,20 A major disadvantage is its low image quality, with poor contrast and low spatial resolution, due to the low extraction rate and high kinetic energy.21
18F-flurpiridaz is a new tracer that is not yet approved for clinical use but is already in phase III clinical trials, with highly promising results.22–25 It is a pyridaben analog that binds to this mitochondrial complex I inhibitor,26–28 and has various characteristics that enable it to provide high-resolution diagnostic images, including high first-pass uptake and slow washout from cardiomyocytes; in addition, the kinetic energy of its positrons is the lowest of all the tracers.10,23,29,30 The main disadvantage of 18F-flurpiridaz is that it must be produced in a cyclotron, although its half-life is the longest of all available tracers (109.8 min), meaning that it can be provided by a regional cyclotron.11 If this tracer performs well in phase III clinical trials and receives FDA approval, it could well become a widely used radiotracer worldwide.
Quantification of absolute myocardial blood flow and myocardial flow reserveBlood supply to the heart is furnished by the epicardial coronary arteries and their branches. These branches become progressively smaller (less than 500 μm) and deeper in the myocardium, forming the microcirculation, where they are the main determiners of microvascular resistance.31 The coronary microcirculation cannot be visualized by coronary angiography, computed tomography angiography or other common imaging methods.32 As a consequence, in contemporary cardiology the possibility of microvascular dysfunction is not generally taken into consideration in therapeutic decision-making.
Nevertheless, although no existing technique is able to visualize the microcirculation in vivo, there are ways to assess parameters that reflect the presence of microvascular dysfunction, including by measuring MBF. This is made possible by the autoregulation of microvascular flow that preserves adequate oxygen supply to the myocardium, by which microvascular resistance is reduced to maintain MBF when coronary arteries are narrowed by atherosclerotic disease.33 At rest, coronary artery stenosis must exceed 85-90% of luminal diameter before MBF is significantly reduced, while under stress, MBF is significantly diminished by 45-50% stenosis.33
Absolute values of MBF in ml/min/g are obtained from time-activity curves based on images of tracer uptake and response of myocardial tissue, which are then adjusted using validated kinetic models specific to each tracer.15,34
In order for MBF quantification to be implemented in clinical practice, automated systems are required that will enable rapid, practical and reproducible analysis of the images obtained from cardiac PET. Several systems for calculating MBF have been developed,15,35,36 each of which uses different methods for segmenting and sampling the activity of the myocardium and the blood pool to calculate the required time-activity curves. Despite these differences, there is a high level of agreement and reproducibility concerning the absolute MBF values produced by these systems.
The MBF values obtained are compared with normal ranges in published databases. Currently accepted normal values range between 2 and 5 ml/min/g in hyperemia. This difference is due to variability in microvascular resistance resulting from factors such as age, gender and cardiovascular risk factors.3–5,37–39 Alternatively, MBF can be expressed on a continuous scale for purposes of diagnosis and prognosis, as well as to support clinical decision-making.40,41
MBF can also be measured by other exams, particularly computed tomography (CT), magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT). Tests of the feasibility and validity of measurements made using these exams have shown them to be viable alternatives to quantification by PET.42–44 However, they suffer from certain disadvantages that limit their widespread application for this purpose. In the case of MRI, these include the need for complex post-processing, its non-volumetric ventricular coverage,45 and the fact that it cannot be used in patients with claustrophobia, implanted electronic devices or severe kidney disease46; CT involves high radiation doses47 and is strongly influenced by heart rate,48 while image resolution in SPECT is low and the technique presents limitations when cardiac output is low.49,50
PET is accordingly considered the gold standard for determining MBF.2,8 Its main advantages over rival exams is short protocol times (30 min)51 that provide images of high spatial and temporal resolution with low radiation doses (1.8 mSv compared to 9.3 mSv for SPECT and 3.7-9.6 mSv for CT).52–54 Nevertheless, PET also has its disadvantages, particularly its high cost, the need for access to a cyclotron to produce 13NH3, H215O and 18F-flurpiridaz,11 and exposure to radiation, unlike MRI.
Another parameter that can be calculated on the basis of MBF is MFR, which represents the proportion of blood flow supplied to the myocardium under stress conditions that is additional to that supplied at rest.55 MFR is affected not only by coronary stenosis but also by various other factors including heart rate, coronary resistance, coronary collateral circulation and coronary vasodilation.55–58
Comparisons with other techniquesThe functional significance of coronary lesions can be assessed through various parameters, including fractional flow reserve (FFR). This is measured invasively using a pressure wire during angiography and is calculated as the ratio between pressure distal and proximal to the stenosis following pharmacologically induced maximum vasodilation, usually with adenosine.59
Lesions with FFR<0.8 or <0.75 are associated with inducible ischemia and are therefore considered hemodynamically significant.60,61 Coronary intervention procedures based on FFR values have been shown to have better results, in terms of preventing cardiovascular events, than those guided by angiography or medical therapy alone.62,63
A new technique for functional assessment has recently emerged as an alternative to FFR, the instantaneous wave-free ratio (iFR). This rapid and simple exam does not require administration of adenosine and is thus highly promising,64–67 either on its own or in hybrid form with FFR,68,69 and is already widely used in catheterization laboratories. Studies comparing the results of iFR and FFR show a high degree of agreement,70–73 although caution should be exercised when using this new technique.74–76
Unlike FFR, which provides a precise measure of lesion severity by assessing alterations in coronary pressures, MFR measures the increase in overall flow in response to vasodilation, either invasively or non-invasively by PET.
Several studies comparing MFR with FFR for functional assessment in CAD have validated the latter,77–79 showing that the former's advantages of being non-invasive, requiring short procedure times and using simple protocols facilitate assessment for both physician and patient.78
An initial study comparing MFR and FFR showed a close correlation between the two in patients with single-vessel disease. However, subsequent studies in multivessel disease showed only modest agreement,80 and also revealed differences in their estimation of the functional significance of coronary stenosis. This discrepancy can be explained by the fact that FFR specifically assesses the epicardial arteries, while MFR is affected not only by epicardial stenosis but also by microvascular dysfunction.77,81
Therefore, in addition to the value of MFR and FFR individually, combining the two parameters may help improve understanding of coronary physiology and offer opportunities to improve patient outcomes by identifying targets for medical therapy and better options for intervention.82
The advantages of myocardial flow reserve in detecting microvascular and balanced multivessel diseaseMyocardial perfusion imaging (MPI) by PET has proved a powerful and versatile diagnostic technique that is able to non-invasively assess the functional significance of coronary lesions. However, this assessment is generally semi-quantitative and hence only relative, and is thus operator-dependent. The exam sets out with the assumption that the region of the myocardium that presents the highest uptake of the perfusion tracer is supplied by visually normal and hence non-obstructed epicardial coronary arteries, and this reference region is then used to evaluate the rest of the myocardium, with regions showing reduced uptake being considered to be supplied by obstructed arteries. This semi-quantitative assessment suffers from certain limitations, particularly its underestimation of the extent of ischemia and obstructive atherosclerosis in the presence of balanced multivessel CAD and its inability to identify patients with microvascular disease who do not present obstructive atherosclerosis.
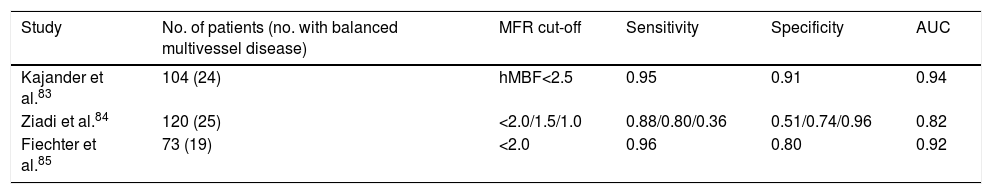
CAD that affects the three main epicardial vessels can lead to reduced perfusion that is balanced across the territories of all three arteries, which can result in an apparently normal MPI result without visually identifiable defects. Various studies have been performed to investigate ways to overcome this limitation of visual or semi-quantitative analysis and to improve diagnostic accuracy in these patients (Table 2).
Diagnostic value of myocardial flow reserve in multivessel coronary artery disease.
| Study | No. of patients (no. with balanced multivessel disease) | MFR cut-off | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| Kajander et al.83 | 104 (24) | hMBF<2.5 | 0.95 | 0.91 | 0.94 |
| Ziadi et al.84 | 120 (25) | <2.0/1.5/1.0 | 0.88/0.80/0.36 | 0.51/0.74/0.96 | 0.82 |
| Fiechter et al.85 | 73 (19) | <2.0 | 0.96 | 0.80 | 0.92 |
AUC: area under the curve; hMBF: hyperemic myocardial blood flow; MFR: myocardial flow reserve.
A 2011 study by Kajander et al. in Turku, Finland, in which absolute MBF was assessed by H215O PET in 104 patients with CAD, concluded that this parameter provided greater diagnostic accuracy than semi-quantitative analysis using the same imaging method, which had a sensitivity of 74% and specificity of 73%, as opposed to 95% and 91%, respectively, for MBF. The authors concluded that quantification of MBF is valuable in the functional assessment of myocardial perfusion with particular importance in multivessel disease, which was diagnosed significantly more often by this technique than by semi-quantitative image analysis.83
A study by Ziadi et al. published in 2012 concluded that MFR<2.0 was an independent predictor of balanced multivessel CAD and was superior to image-based relative assessment. This study, carried out at the University of Ottawa Heart Institute in 120 patients with CAD documented or suspected following angiography and assessed by 82Rb PET, also demonstrated that MFR is significantly lower in patients with three-vessel disease than in those without, with a diagnostic sensitivity of 88%.84
Also in 2012, a study of 73 patients by Fiechter et al. in Zurich using 13NH3 PET further demonstrated the superior diagnostic value of MFR in CAD compared to semi-quantitative analysis. In this study, 30% of the patients initially classified as not having CAD on the basis of a semi-quantitative analysis were diagnosed with multivessel disease according to MFR, which increased diagnostic sensitivity from 79% to 96%.85
The studies summarized in Table 2 indicate that the diagnostic value of absolute MFR measurement is higher than that of qualitative or semi-quantitative assessment, which is the most common method used in most contemporary cardiology laboratories performing cardiac PET to assess patients with suspected or confirmed CAD. The findings of all of these studies have an area under the curve >0.80 (>0.90 in two). Since this statistic includes both sensitivity and specificity and thus reflects overall accuracy, these studies confirm that assessment of MFR has high diagnostic value for the detection of balanced multivessel disease. They also show that MFR has higher sensitivity and specificity than semi-quantitative analysis, further demonstrating its diagnostic potential in these patients. Nevertheless, given that there are some differences in the results obtained, further studies are called for that will enable the incremental value of MFR in this setting to be determined in a clear and consistent fashion.
Microvascular disease in the absence of obstructive atherosclerotic disease of the epicardial coronary arteries poses another enormous challenge for the cardiologist, since the non-invasive exams used in clinical practice cannot identify its presence through visual analysis alone. Microvascular disease is in fact the first stage in the progression of CAD, and so its detection can help maximize the ability to diagnose the disease early.
Several studies have been performed to determine whether diagnosis of CAD at an early stage, when no significant alterations are detectable on angiography or semi-quantitative MPI, can help provide better risk stratification and prognostic assessment in these patients. None of the studies included patients with previous myocardial infarction, prior cardiac revascularization or known valve disease.
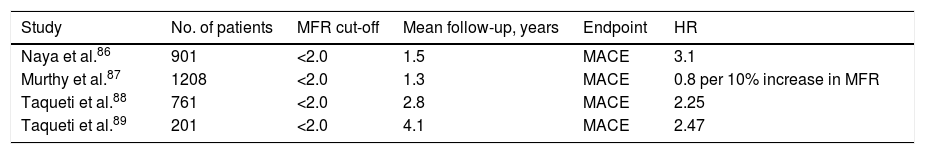
A study by Naya et al. published in 2013 of 901 individuals with suspected CAD and normal MPI PET scan demonstrated that the presence of impaired MFR, rather than the extent of coronary calcium deposits, significantly enhanced risk stratification in these patients. Their analysis showed that for any coronary calcium score, the presence of reduced MFR (>2) was consistently associated with a higher rate of major adverse cardiac events (MACE), reflecting the combined effects of diffuse non-obstructive atherosclerosis and coronary microvascular dysfunction on the prognosis of these patients.86
In 2014, a study by Murthy et al. was published on a cohort of 1218 individuals with suspected CAD and normal PET MPI. Their analysis showed that impaired MFR (>2) was independently associated with MACE, and also that this phenotype was common, being observed in over 50% of patients.87
In 2015, Taqueti et al. published results of a study on 761 patients with chest pain or dyspnea and intermediate pretest probability of having CAD, but with MPI within normal limits by semi-quantitative PET analysis. The authors concluded that impaired MFR (<2) was independently associated with MACE and with positive troponin, and that as an indicator of microcirculatory dysfunction it was a better marker for MACE than positive troponin. Patients with both positive troponin and impaired MFR had the highest risk of MACE.88
Finally, another study by the same team published in late 2017 analyzed 201 patients with suspected CAD but without flow-limiting stenosis or reduced left ventricular ejection fraction, and with PET MPI within normal limits, over a mean follow-up of four years. The study's primary aim was to determine whether MFR was of additional value for diagnosing microvascular disease and could thus contribute to risk stratification for MACE, hospitalization for heart failure with preserved left ventricular ejection fraction, and diastolic dysfunction. Impaired MFR was independently associated with all these outcomes.89
The studies summarized in Table 3 reveal the added value that MFR can provide in the detection of CAD in its early stages by identifying patients with microvascular disease. All the results with an MFR cut-off of <2 and MACE as endpoint showed hazard ratios of >1, meaning that this parameter is consistently associated with worse prognosis. Quantification of MFR in these patients thus enables a more detailed characterization and risk stratification of CAD.
Diagnostic and prognostic ability of myocardial flow reserve in microvascular coronary disease.
| Study | No. of patients | MFR cut-off | Mean follow-up, years | Endpoint | HR |
|---|---|---|---|---|---|
| Naya et al.86 | 901 | <2.0 | 1.5 | MACE | 3.1 |
| Murthy et al.87 | 1208 | <2.0 | 1.3 | MACE | 0.8 per 10% increase in MFR |
| Taqueti et al.88 | 761 | <2.0 | 2.8 | MACE | 2.25 |
| Taqueti et al.89 | 201 | <2.0 | 4.1 | MACE | 2.47 |
HR: hazard ratio; MACE: major adverse cardiac events; MFR: myocardial flow reserve.
Further studies are required to determine how to select patients for this quantification, in order to develop simple and feasible algorithms for use in clinical practice. At the same time, changes in the cost/benefit ratio of PET for quantification of MBF and MFR will be crucial in the future for the more widespread implementation of this technology worldwide.
Final considerationsMortality due to CAD remains high, despite advances in treatment and prophylactic interventions. Accurate diagnosis through methods that assess not only anatomy but also functional repercussions, especially in the early stages of the disease, is thus a promising way to reduce the number of deaths associated with this disease.
Quantification of MBR and MFR by PET has been shown to provide potential advantages in the characterization and diagnosis of CAD, particularly for balanced multivessel disease and microvascular disease.
Despite its high diagnostic accuracy for assessment of ischemia, the use of cardiac PET for absolute quantification of MBF is limited by the availability of the technique. Further studies are required to identify which patients will benefit most from this exam.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernandes J, Ferreira MJ, Leite L. Quantificação do fluxo sanguíneo miocárdico por tomografia por emissão de positrões – Atualização. Rev Port Cardiol. 2020;39:37–46.