Despite rapid advances in transcatheter aortic valve prostheses, anatomical constraints remain that can limit access to this treatment for patients with severe aortic stenosis. The objective of this study was to determine the proportion of patients anatomically suitable for this technique using the different devices and approaches available.
MethodsWe retrospectively analyzed 145 consecutive patients referred to our center for transcatheter aortic valve implantation. Aortic annulus diameter was measured by transesophageal echocardiography and minimum iliofemoral diameter was determined by multidetector computed tomography. We determined the proportion of patients anatomically suitable for current devices (26-mm, 29-mm and 31-mm Medtronic CoreValve for transfemoral, transaxillary or transaortic approaches, and 23-mm, 26-mm and 29-mm Edwards Sapien XT for transfemoral or transapical approaches).
ResultsThe Medtronic CoreValve was suitable for 89% of patients via transfemoral access and 93.8% via transaxillary or transaortic approaches, while the Edwards Sapien XT was suitable for 82.1% of patients via transfemoral and 97.2% via transapical approaches. Only 1.4% of patients were anatomically unsuitable for all devices and approaches.
ConclusionsIn this population, most patients were anatomically suitable for transcatheter aortic valve implantation if assessed on the basis of multiple devices and multiple access approaches.
Apesar de a rápida evolução das próteses valvulares aórticas percutâneas, persistem restrições anatómicas que podem limitar o acesso dos doentes com estenose aórtica severa a este tratamento. O objetivo deste estudo foi determinar a proporção de doentes anatomicamente adequados para os diferentes dispositivos e acessos, numa população candidata a este tratamento.
MétodosAnálise retrospetiva de 145 doentes consecutivos referenciados ao nosso centro para implantação de válvula aórtica percutânea. A dimensão do anel aórtico foi determinada por ecocardiograma transesofágico e o diâmetro mínimo das artérias iliofemorais foi obtido por tomografia computadorizada multidetetores. Foi determinada a proporção de doentes anatomicamente adequados para as próteses actualmente disponíveis (Medtronic CoreValve de 26, 29 e 31mm por acesso transfemoral, transaxilar ou transaórtico; Edwards Sapien XT de 23, 26 e 29mm por acesso transfemoral ou transapical).
ResultadosDos doentes avaliados, 89% eram adequados para as próteses Medtronic CoreValve por via transfemoral e 93,8% eram adequados para abordagem subclávia ou transaórtica. Em relação às próteses Edwards Sapien XT, 82,1% eram adequados para acesso transfemoral e 97,2% eram adequados para a via transapical. Apenas 1,4% dos doentes não apresentavam anatomia viável para esta técnica considerando todos os dispositivos e abordagens possíveis.
ConclusõesNesta população, a maioria dos doentes foi considerada anatomicamente adequada para tratamento percutâneo, numa estratégia multi-dispositivo e multi-abordagem.
Transcatheter aortic valve implantation (TAVI) has been shown to be effective and safe in the treatment of patients with severe aortic stenosis and high surgical risk.1–8 The latest generation prostheses available in Europe are the Medtronic CoreValve (Medtronic Inc., Minneapolis, MN) and the Edwards Sapien XT (Edwards Lifesciences Inc., Irvine, CA). The Medtronic CoreValve is a self-expanding device, available in 26-mm, 29-mm and 31-mm sizes, and can be implanted via a transfemoral, transaxillary/subclavian or transaortic approach. The Edwards Sapien XT is a balloon-expandable valve, available in 23-mm, 26-mm and 29-mm sizes, to be implanted via a transfemoral or transapical approach. Despite rapid advances in these devices, anatomical constraints remain, particularly with regard to the diameter of the aortic annulus (for all approaches) and of the iliofemoral arteries (for transfemoral approach), which can limit patient access to this treatment. Our objective was to determine the proportion of patients referred for TAVI who were anatomically suitable for the technique using the latest devices and the various approaches available.
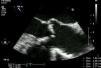
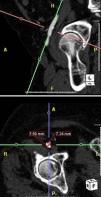
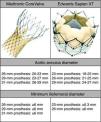
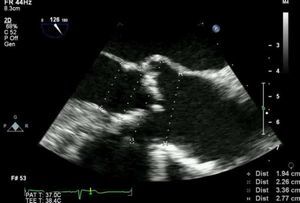
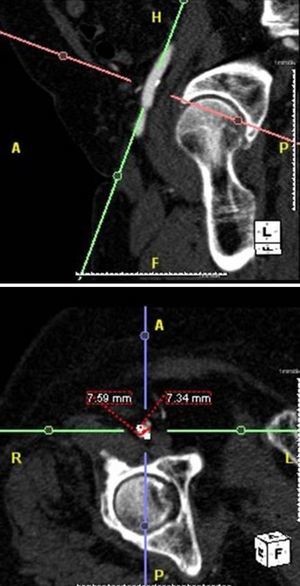
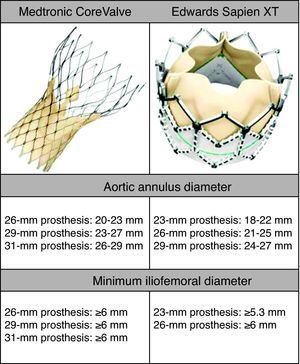
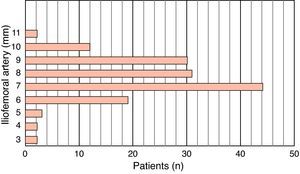
MethodsWe retrospectively analyzed 145 consecutive patients referred to our center for TAVI between March 2007 and October 2011. All patients were assessed by transesophageal echocardiography (TEE) and multidetector computed tomography (MDCT). The aortic annulus diameter obtained by TEE in long-axis view of the left ventricle at 120–140° (Figure 1) was used whenever possible. Minimum iliofemoral diameters were determined by MDCT for the entire segment proximal to the head of the femur, the diameter selected being that of the artery with the most favorable anatomy (Figure 2). The proportion of patients considered suitable for the various devices and approaches was determined according to their respective anatomical requirements (Figure 3).
The aortic annulus diameters required for the Medtronic CoreValve are 20–23mm for the 26-mm, 23–27mm for the 29-mm, and 26–29mm for the 31-mm valve. A further requirement is that the diameter of the ascending aorta be ≤40mm for the 26-mm, and ≤43mm for the 29-mm and 31-mm prostheses. An 18F introducer is used for transfemoral access, which requires a minimum iliofemoral diameter of 6mm. Alternatively, the prosthesis can be delivered via the subclavian artery (also requiring a minimum diameter of 6mm) or directly via the ascending aorta.
The required aortic annulus diameters for the Edwards Sapien XT are 18–22mm for the 23-mm, 21–25mm for the 26-mm, and 24–27mm for the 29-mm valve (the latter is currently available only for a transapical approach). Transfemoral access using the new Edwards eSheath delivery system with a dynamic expansion mechanism requires a minimum iliofemoral diameter of 5.3mm for 23-mm/16F systems and 6mm for 26-mm/18F systems. Alternatively, the device can be implanted using a transapical approach.
The proportion of patients anatomically suitable for the various devices and approaches was compared using the McNemar test.
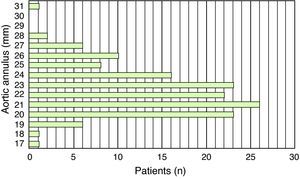
ResultsThe study population consisted of 145 patients, of whom 70 were male (48.3%), with a mean age of 78±7.1 years. Mean aortic annulus diameter was 22.5±2.4mm and mean minimum iliofemoral diameter was 7.7±1.5mm (Figures 4 and 5).
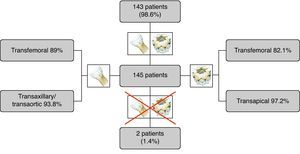
Of the 145 patients, 129 (89%) were suitable for Medtronic CoreValve prostheses via transfemoral access and 136 (93.8%) were suitable for transaxillary or transaortic approaches; with regard to Edwards Sapien XT devices, 119 patients (82.1%) were suitable for transfemoral and 141 (97.2%) for transapical approaches. Of nine patients who were anatomically unsuitable for Medtronic CoreValve prostheses, seven could be treated with Edwards Sapien XT devices, while of four unsuitable for Edwards prostheses, two were treatable by CoreValve devices. Only two patients (1.4%) were anatomically unsuitable for TAVI using any device or approach (Figure 6). A similar proportion of patients were suitable for CoreValve or Edwards devices (93.8% vs. 97.2%, p=0.1797). Assessment on the basis of multiple devices increased the proportion of the study population with suitable anatomy from 93.8% to 98.6% (p=0.016) for the Medtronic CoreValve, and from 97.2% to 98.6% (p=0.5) for the Edwards Sapien XT. The proportion of patients treatable by transfemoral access on the basis of multiple devices was 93.8%, significantly higher than the 89% with Medtronic CoreValve (p=0.016) and the 82.1% with Edwards Sapien XT (p<0.001) valves. The proportion of patients anatomically suitable for TAVI on the basis of multiple devices and multiple access approaches was 98.6%, compared to 93.8% (p=0.0156) with multiple devices via transfemoral access only.
Of the 145 patients assessed, 72 had undergone TAVI up to October 2011; of these, 71 were implanted with Medtronic CoreValve devices (60 via transfemoral, nine via subclavian/transaxillary and two via transaortic approaches) and one patient was treated with an Edwards Sapien XT valve.
DiscussionThe study considered only two anatomical criteria – aortic annulus diameter and iliofemoral artery diameter – since these dictate the eligibility of patients for TAVI and the approach to adopt. Nevertheless, there are other anatomical aspects that need to be considered, including the presence of severe left ventricular hypertrophy, small sinuses of Valsalva, and excessive calcification or tortuosity of the iliofemoral arteries.9 These factors are not absolute exclusion criteria but they will affect procedure success and complication rate, as well as increasing risk, and should thus be assessed on a case-by-case basis.
Determining aortic annulus diameter is an essential step in evaluating candidates for TAVI, since it may immediately exclude a patient from the procedure or dictate the type of device to be implanted. It must therefore be measured accurately, since it determines the choice of the most appropriate prosthesis in each case to minimize the risk of paravalvular leak and device migration.10 There is currently no gold standard exam for annulus measurement, which can be performed by transthoracic echocardiography (TTE), TEE, MDCT or calibrated aortography,10,11 but the few studies comparing the different methods have conflicting results. One limitation of two-dimensional echocardiography is that measurements are based on a single view and assume that the aortic annulus is circular. However, MDTC studies have demonstrated that the annulus is often oval, with significant differences between minimum and maximum diameters.12 In general, the aortic annulus diameter is greater when assessed by MDTC than by TEE, and the latter is in turn greater than that assessed by TTE.10,12–14 In current clinical practice, the eligibility of patients for TAVI and the choice of prosthesis size are generally based on TEE measurement since it is the standard technique and has shown good results.11,13 Recent studies on three-dimensional (3D) imaging have shown a good correlation between measurements obtained by 3D TEE and those assessed by MDCT, which makes echocardiographic assessment a more viable option in these patients.15,16
Another essential step in evaluating these patients is assessment of the peripheral arterial system, which determines the approach to adopt. This can be performed by MDTC, peripheral angiography or magnetic resonance imaging with gadolinium.10 MDTC is a non-invasive technique that provides good quality images of the vascular system through cross-sectional views and 3D reconstructions, which help in procedure planning.10
Nearly all patients (98.6%) in our study population were considered anatomically suitable for TAVI based on all the prostheses and approaches available. Another important finding was that most patients (93.8%) could be treated via transfemoral access, the preferred approach for any percutaneous procedure.
Although each device was able to treat a large number of patients, assessment on the basis of multiple devices further extended the range of treatable patients. Replacement valves are now more similar in terms of anatomical requirements compared to earlier devices. Nevertheless, there are still important structural differences between them, which can prompt the choice of one over another according to individual patient characteristics. Marked angulation of the ascending aorta or aortic arch may be more suited to anterograde (transapical) delivery of the prosthesis,9 as would a markedly sigmoid septum10; in cases of low ostial implantation of the coronary arteries, it is safer to use a self-expanding prosthesis.9 As more experience is gained of the various devices and approaches, it will be possible to tailor the choice of prosthesis and approach to individual patients.
A study published in 2010 of 100 candidates for TAVI demonstrated that 89% were suitable for CoreValve and 88% for Edwards devices,9 but when assessed for transfemoral access only, 84% were treatable with CoreValve and 28% with Edwards prostheses. The study assessed anatomical suitability based on the devices available at that time (26-mm and 29-mm CoreValve with 18F transfemoral access, and 23-mm and 26-mm Edwards Sapien, with 22F and 24F femoral access, respectively).
Recent years have seen rapid developments in percutaneous aortic valve prostheses, with new sizes and smaller-profile delivery systems, with the result that a greater number of patients are now considered anatomically suitable for the technique.
Besides anatomical features, other factors affect eligibility for TAVI and mean that many patients will be poor candidates. Moderate to severe mitral regurgitation or low ejection fraction with no contractile reserve, although they should be assessed on a case-by-case basis, are generally exclusion criteria. In addition, many of these patients are elderly and frail in poor general health, or have major comorbidities that will affect their short-term survival. Since current devices have fewer anatomical constraints, such clinical aspects are now the main factors affecting patient access to percutaneous treatment. Even so, ongoing technological advances in this area are likely to lead to a new generation of devices that will overcome the remaining anatomical limitations, simplify the procedure and minimize complications.
Up until October 2011, 72 of the 145 patients assessed at our center had undergone TAVI. We cannot be certain that 98.6% were anatomically suitable for the technique at the time of referral to our center, since in most cases not all the devices considered in this study were on the market. For example, the Edwards Sapien XT prosthesis was only available in our center from July 2011 and the transapical approach was only implemented in 2012. In addition, some of these patients are on the waiting list for the procedure. Lastly, despite being anatomically suitable for TAVI, many patients present the clinical characteristics mentioned above (frailty, poor general health and comorbidities) which make them poor candidates for the technique.
ConclusionsIn this population, 98.6% of the patients were considered anatomically suitable for TAVI using the devices and approaches currently available, and 93.8% could be treated via transfemoral access. Rapid advances in these devices have overcome most of the initial anatomical constraints, significantly extending the range of candidates for the treatment. At present, clinical rather than anatomical characteristics appear to be the main factor affecting access to percutaneous treatment for most patients with severe aortic stenosis and high surgical risk.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sousa O, et al. Implantação percutânea de válvula aórtica: a anatomia é (ainda) o fator limitante? Rev Port Cardiol. 2013. doi:10.1016/j.repc.2012.08.009.