Transcatheter aortic valve implantation (TAVI) is the standard treatment for patients with severe aortic stenosis and unacceptable surgical risk. These are usually elderly patients with multiple comorbidities.
We report the case of a 20-year-old man with mandibuloacral dysplasia, an extremely rare premature aging syndrome, and severe symptomatic aortic stenosis, referred to our center for TAVI after being considered unsuitable for surgical aortic valve replacement. TAVI by a transfemoral approach was performed successfully. Severe acute respiratory failure that did not respond to optimal conventional treatment led us to employ venovenous extracorporeal membrane oxygenation. The device was removed after 10 days, and the patient was discharged home 27 days later. At one-year follow-up he is in NYHA class I with full functional autonomy. To the best of our knowledge, this is the youngest patient to undergo TAVI reported in the literature.
A implantação percutânea de prótese valvular aórtica (TAVI) é, atualmente, o tratamento de eleição para doentes com estenose aórtica grave sintomática e risco cirúrgico inaceitável. Tratam-se habitualmente de doentes com idade avançada e múltiplas comorbilidades. Os autores apresentam o caso de um doente de 20 anos de idade, portador de displasia mandíbuloacral, uma síndrome «progeria-like» extremamente rara, com estenose aórtica grave sintomática, referenciado ao nosso serviço para TAVI após ter sido recusado para cirurgia de substituição valvular. Foi implantada prótese valvular aórtica por via femoral, sem intercorrências de relevo. Por insuficiência respiratória aguda grave com falência da abordagem terapêutica convencional foi necessária a implantação de Extracorporeal membrane oxygenation (ECMO) veno-venoso. O suporte com ECMO foi mantido durante dez dias e o doente teve alta para o domicílio 27 dias depois. No seguimento a um ano, o doente encontra-se em classe funcional I da NYHA, com autonomia nas atividades da vida diária. Após revisão da literatura, concluímos tratar-se do doente mais jovem alguma vez tratado por TAVI.
Transcatheter aortic valve implantation (TAVI) is the standard treatment for patients with severe aortic stenosis who are considered unsuitable or high-risk for surgical valve replacement following analysis of technical feasibility, life expectancy and quality of life.1,2 These are usually elderly patients (mean age >80 years in large multicenter registries) with multiple comorbidities.2–7
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is an effective and well-tolerated therapeutic option in patients with severe respiratory failure of potentially reversible etiology.8–10 The Conventional ventilatory support versus Extracorporeal membrane oxygenation for Severe Adult Respiratory failure (CESAR) trial, published in 2009, demonstrated a positive prognostic impact in such patients treated in specialist centers.10
Case reportA 20-year-old male university student living independently, with mandibuloacral dysplasia (a rare autosomal recessive syndrome characterized by premature aging, bone deformities, skin atrophy and lipodystrophy), was referred to our center for severe symptomatic aortic stenosis (peak and mean gradients of 88 mmHg and 60 mmHg, respectively, and functional valve area of 0.7 cm2). He presented symptoms of worsening heart failure, in New York Heart Association (NYHA) functional class III.
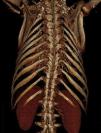
He was refused surgical valve replacement by two referral centers due to thoracic deformation with restrictive ventilatory abnormalities and multiple comorbidities, including hypertension, type 2 diabetes, dyslipidemia, bilateral carotid stenosis (50%), thrombosis of the right subclavian artery, obstructive sleep apnea requiring bilevel positive airway pressure (BiPAP) support and proliferative mesangial glomerulonephritis (creatinine clearance 123 ml/min) (Figure 1).
Following evaluation by our center's heart team, the patient was accepted for TAVI.
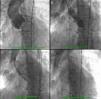
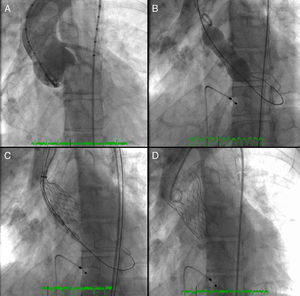
A 26-mm aortic valve prosthesis (Medtronic CoreValve™) was implanted using a transfemoral approach, with an excellent final angiographic result (Figure 2). The procedure was performed under general anesthesia and VMI following elective orotracheal intubation, guided by bronchofiberscopy due to the patient's facial deformities and problematic airway. Weaning from ventilatory support was rapid, with extubation within 24 hours. A setting of type 2 respiratory failure and fever began on the first postoperative day; non-invasive ventilation with BiPAP was instituted and blood samples collected for testing, which was negative. A chest X-ray revealed infiltrate in the left lung base and widespread nodular lesions in cervical and thoracic soft tissues, associated with lipodystrophy. Empirical antibiotic therapy was begun with levofloxacin. Laboratory tests showed leukocytosis (27.7×109/l) with neutrophilia and C-reactive protein of 10.4 mg/dl.
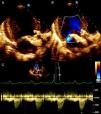
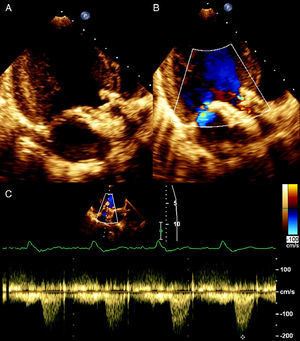
Transthoracic echocardiography showed the aortic prosthesis to be correctly positioned, with a peak transvalvular gradient of 17 mmHg, a small central regurgitant jet, and preserved left ventricular systolic function (Figure 3).
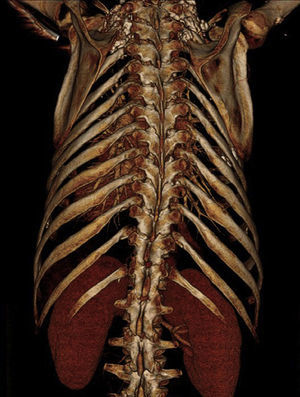
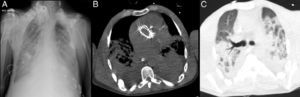
Rapid clinical deterioration ensued, with severe respiratory failure refractory to optimal medical therapy and non-invasive ventilation (blood gases: pH 7.14; pCO2 74 mmHg; pO2 32 mmHg; SO2 41%; and lactates 5.4 mmol/l). Since it was impossible to intubate and ventilate the patient, even after several attempts at orotracheal intubation guided by bronchofiberscopy, emergency percutaneous tracheotomy was performed. Antibiotic therapy was changed to vancomycin and imipenem, and inotropic support was begun due to hypotension refractory to fluid administration. The chest X-ray revealed diffuse infiltrate and thoracic computed tomography showed foci of bilateral parenchymal consolidation, ground-glass opacification and bilateral pleural effusion (Figure 4).
Chest X-ray showing bilateral parenchymal infiltrate and nodular lesions in soft tissues associated with lipodystrophy (A); thoracic computed tomography at the level of the aortic prosthesis, mediastinal window (B) and lung window (C), showing foci of bilateral parenchymal consolidation, ground-glass opacification and bilateral pleural effusion.
On the fourth day after TAVI, the patient developed acute respiratory distress syndrome (ARDS) (pO2/FiO2 ratio: 41) refractory to medical therapy, ventilatory optimization and alveolar recruitment maneuvers; since this was of potentially reversible etiology, an ECMO device was implanted via cannulation of the left femoral and right jugular veins (16 Fr cannulas), without complications. Improvement was seen in clinical and blood gas parameters (initial output of 2.5 l/min), which enabled ventilatory and inotropic support to be considerably reduced. Pseudomonas aeruginosa (susceptible to piperacillin-tazobactam) was subsequently isolated in tracheobrochial secretions.
VV-ECMO support was maintained, with no vascular complications. The patient developed heparin-induced thrombocytopenia, and so unfractionated heparin was replaced by lepirudin and platelet transfusion, and acute anemia due to hemolysis, requiring transfusion of four units of red cell concentrate. The ECMO device was removed after 10 days, invasive mechanical ventilation was continued for a further three days, and then the tracheotomy cannula was removed. The patient was discharged 27 days after removal of the ECMO device and referred for a home rehabilitation program (due to marked muscle wasting and loss of 10 kg during hospitalization, minimum weight 27 kg) and respiratory therapy.
At two-month follow-up, the patient was in NYHA class I, with functional autonomy, and had returned to his studies. At one year, he remains asymptomatic and the prosthetic valve is functioning normally, with no significant regurgitation.
DiscussionThere were various difficulties in this case, particularly regarding acceptance of the patient for TAVI and treatment of associated complications, as well as underlying ethical questions.
Selecting a patient for TAVI involves four steps: (1) confirmation of the severity of aortic stenosis; (2) evaluation of symptoms; (3) analysis of surgical risk, life expectancy and quality of life; and (4) assessment of feasibility and exclusion of contraindications.1 The third step raised various questions, given the rarity of the patient's clinical condition and his associated comorbidities, which are not included in conventional risk scores such as the EuroSCORE and Society of Thoracic Surgeons (STS) score, and the fact that the life expectancy of these patients is not known. However, since the patient was active with a good quality of life before the onset of symptoms and had been refused aortic valve replacement surgery, he was accepted for TAVI after evaluation by our multidisciplinary team.
The decision to use VV-ECMO as a rescue therapy for severe ARDS took account of its potential reversibility; it proved to be effective and safe, in accordance with our own experience and the literature.
Following a review of the literature, this patient appears to be the youngest to undergo TAVI, no similar patients being included in the numerous published registries and randomized trials, which further increases the rarity of the case.
The successful outcome in the present case highlights the need for complex interventional cardiology procedures to be performed in centers with intensive care units that have adequate resources to deal with possible complications. Cardiologists in this type of unit must be familiar with the latest techniques available for hemodynamic and respiratory support.
ConclusionThe case presented is particularly interesting due to the rarity of the patient's underlying disease and the fact that he is the youngest to undergo TAVI. It illustrates the technical and ethical challenges posed when intervening in increasingly complex patients, highlighting the importance of heart teams with different specialties, particularly intensive cardiologists, for the success of such procedures.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Please cite this article as: Faria R, Caeiro D, Fontes de Carvalho R, et al. Implantação percutânea de válvula aórtica em doente muito jovem. Rev Port Cardiol. 2013;32:827–831.