Riata and Riata ST silicone defibrillation leads are prone to externalization of conductors due to inside-out abrasion in the high-voltage system, causing structural damage which may be accompanied by electrical failure. These situations are easily detected by fluoroscopy or radiology and by inspection of intracardiac electrograms and/or measurement of impedance. However, older pulse generators do not automatically perform all the measurements needed to assess the integrity of the high-voltage electrical system, nor do they have patient notifier alerts in case of dysfunction.
The authors describe the case of a patient in whom structural damage was detected on fluoroscopy during pulse generator replacement. They discuss the best strategy in these patients, considering current knowledge of this dysfunction.
Os elétrodos de desfibrilhação Riata e Riata ST, com revestimento de silicone, são propensos a exteriorização dos fios condutores por mecanismo de abrasão interna-externa dos fios condutores do sistema de alta voltagem, provocando uma alteração estrutural que pode, ou não, ser acompanhada de alterações elétricas. Ambas as situações são facilmente detetadas, quer por fluoroscopia/radiologia quer por inspeção do traçado endocavitário e/ou medição das impedâncias. No entanto, geradores de famílias mais antigas não fazem automaticamente todas as medições necessárias para aferir a integridade elétrica do sistema de alta voltagem nem possuem sistemas de notificação do doente em caso de disfunção.
Descreve-se o caso de um doente cuja alteração estrutural foi detetada na radioscopia aquando da substituição do gerador em exaustão. Discute-se qual a melhor estratégia nestes doentes, face ao atual conhecimento desta disfunção.
ethylene tetrafluoroethylene
elective replacement indicator
Food and Drug Administration
implantable cardioverter-defibrillator
right ventricle
superior vena cava
Riata and Riata ST silicone defibrillation leads (St. Jude Medical) were marketed from 2002 and 2005, respectively, until 2010. Around 227000 were sold worldwide, and around about 79000 remain active in the USA alone.1 The original 8F Riata leads have an inner coil containing the pacing and sensing electrodes and two lumens (single coil) or three lumens (dual coil) equidistant from the center, through which pass pairs of cables carrying current to the high-voltage circuit, insulated with ethylene tetrafluoroethylene (ETFE). Reducing inner coil and lumen diameter, but maintaining the thickness of the silicone insulation, enabled the lead diameter to be reduced to 7F (Riata ST).1
Initial performance was excellent, but in 2008 the first cases of structural problems appeared.2 The design of the leads meant that the movement induced by tricuspid valve closure caused inside-out abrasion, particularly between the two defibrillation coils or in the proximal part (located in the right atrium) of single-coil systems. This abrasion can lead to externalization of conductors outside the lead insulation body and exposure of ETFE. The reported time between implantation and detection of the problem varies considerably, between 27 months3 and 79±14 months.4
Such structural damage can occur in isolation, without any changes in lead function, and may only be detected by chest X-ray or fluoroscopy.3 However, cases have been described of electrical dysfunction (alterations in electrograms or impedances, particularly in the high-voltage system) when externalized conductors contact neighboring coils; this can lead to defibrillation failure and possibly death. As a result, on December 15, 2011, the US Food and Drug Administration (FDA) issued a Class I recall on the device because of the potential risk of serious injury or patient death if affected devices malfunction.5
The manufacturer, St. Jude Medical, has published ongoing recommendations for dealing with the situation, including establishing a dedicated website (www.riatacommunications.com). Their attitude has mainly been conservative but aims at minimization of risks and early detection of possible dysfunction.1,3,6
Nevertheless, many doubts remain concerning the behavior of externalized leads with exposed ETFE during defibrillation, as well as the possibility of rupture and thrombus formation, and there is agreement that decisions should be individualized with the informed participation of the patient.
Case reportAn 81-year-old patient with non-ischemic heart disease and left ventricular dysfunction (ejection fraction 30%) had a single-chamber implantable cardioverter-defibrillator (ICD) (Epic Plus VR V-196, Riata 1570 8F double-coil lead) implanted on March 15, 2005 for secondary prevention due to syncopal ventricular tachycardia. The right ventricular (RV) threshold was 0.4 V at 0.4 ms, R wave 12.5 mV, pacing impedance 864 Ω and shock impedance, measured during defibrillation testing, 44 Ω.
The patient was followed regularly in the cardioverter-defibrillator clinic. Three episodes of sustained monomorphic ventricular tachycardia were recorded, converted by antitachycardia pacing; there were no episodes of cardioversion/defibrillation.
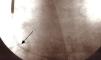
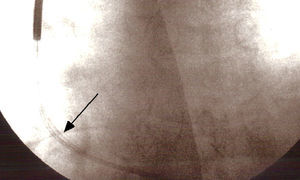
Eighty-seven months after implantation the elective replacement indicator (ERI) was reached and pulse generator replacement was accordingly scheduled. Fluoroscopy performed prior to replacement to verify lead integrity revealed an externalized conductor visible outside the shadow of the lead (Figure 1).
All of the parameters testable by the programmer were normal; this model does not measure the impedance of the high-voltage shock circuit. Careful observation of the image showed that the externalization was relatively slight, no loops or angulations being visible, and so a conservative approach was adopted, initially only replacing the pulse generator. During the intervention, after generator replacement, the high-voltage circuit impedance was measured and the intracavitary electrograms (RV-generator, superior vena cava [SVC]-generator, RV-SVC) were subjected to lengthy and meticulous examination, which showed them to be normal. Accordingly, as there was no evidence of electrical dysfunction and the new pulse generator had the ability to perform periodic tests of shock impedance, it was decided to keep the original lead and to conclude the intervention. Defibrillation testing was not considered appropriate. Early detection of dysfunction was programmed and the patient and his family were advised what to do if the ICD's vibratory patient notifier was triggered.
DiscussionAlthough there are no definitive data on the behavior of Riata or Riata ST leads with externalized ETFE-coated conductors, no fatal complications have been reported resulting solely from failure of lead integrity.7 If the abrasion progresses to the inside of one of the coils, however, this can lead to rupture of the insulation, short-circuits in the defibrillation system and failure to deliver therapy, without lead externalization being observed. In such cases the dysfunction can only be detected through the presence of electrical noise in intracardiac electrograms, including in the coils, or through changes in impedance in the high-voltage system.7,8
The incidence of structural failure in 8F Riata leads has been reported as 12–33%, and is higher in single-coil models,9–11 in which 20% of affected electrodes present electrical dysfunction,8 although doubts have been expressed concerning these figures due to under-reporting and the low number of leads explanted and returned to the manufacturer for analysis.3 The results of phase I (externalizations only, not analyzing electrical dysfunction) of the Riata Lead Evaluation Study (North America), published in July 2012, show a statistically significant difference in overall externalization rates between the 8F and 7F leads (17.9% vs. 9.4%, p=0.02), even with leads implanted less than six years previously, in both dual- and single-coil models (dual-coil 23.3% vs. 10.4%, p<0.001, single-coil 28.9% vs. 4.3%, p=0.001).12,13
The manufacturer's recommendations,6 which are in line with those of the FDA,5 can be summarized as follows:
- 1)
Perform initial imaging (two-view chest X-ray – posteroanterior and lateral – or three-view fluoroscopy (posteroanterior, right anterior oblique at 45°, and left anterior oblique at 45°);
- 2)
Aggressively monitor the parameters most likely to show changes, including pacing impedance outside the range 200–1000 Ω and shock impedance more than 25 Ω above or below normal values, very short intervals (<250 ms) on the RV coil intracavitary electrogram, R-wave variations, variations in AutoCapture threshold, or increase in number of intervals needed for detection from 24 to 30;
- 3)
Ensure that the vibrating patient notifier is programmed;
- 4)
Base the final decision on the clinical context and take into consideration the patient's informed opinion
- •
If no structural or electrical problems are detected, the normal three-monthly follow-up should be continued.
- •
In the event of structural damage but no electrical dysfunction, with low-risk patients (who have not required defibrillation therapy and are not pacing-dependent) who are socially and psychologically able to deal with the situation, prophylactic replacement is not indicated. Three-monthly follow-up should be continued, possibly with annual monitoring using imaging methods appropriate for the patient's risk profile; defibrillation testing is not indicated.
- •
When there is electrical dysfunction, or if the patient is high-risk or makes an informed decision for replacement, the lead should immediately be replaced. Routine lead explantation is not indicated due to the risk of fracture of the weakened device, although this can be successfully achieved.14
However, there is no mention either in the literature or in the published guidelines concerning the appropriate approach to patients with older ICDs that do not automatically perform impedance checks or are not equipped with vibratory patient notifiers (Epic, Epic Plus, Atlas and Atlas Plus), which in our cardiology department still account for 31% of patients with Riata and Riata ST leads. Given the importance of monitoring the high-voltage circuit, we consider it prudent to replace the lead (especially in high-risk patients) if the pulse generator has more than six months before its ERI and to replace the generator if its ERI is imminent.
Remote monitoring via Merlin.net would bring additional benefits but is not yet available.12
Phase II of the Riata Lead Evaluation Study, which aims to determine the incidence of electrical lead dysfunction with and without lead externalization, is about to begin, with a minimum follow-up of two years.5
ConclusionsAlthough the actual prevalence of compromised lead integrity due to inside-out abrasion and conductor externalization in Riata and Riata ST leads, and the extent of the resulting electrical dysfunction, are unknown, all centers that have implanted ICDs using these leads will have to deal with the problem with increasing frequency. Isolated externalization can be treated conservatively so long as the programming and monitoring indications published by St. Jude Medical and the FDA are followed, as in the case presented.
The authors have adopted a systematic approach to the different types of situations, and propose a rational way to deal with older ICDs which is not included in the published recommendations.
Conflict of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Please cite this article as: Madeira J, Parreira L, Amador P, et al. Falha de integridade dos elétrodos de desfibrilhação Riata e Riata ST: um problema atual. Rev Port Cardiol. 2013;32:823–826.