Ventilatory efficiency, evaluated by cardiopulmonary exercise testing (CPET), has considerable prognostic value in patients with chronic heart failure (CHF) due to left ventricular systolic dysfunction (LVSD). Its determinants nevertheless remain controversial.
AimTo investigate the possible correlation between parameters of ventilatory efficiency obtained by CPET and thoracic fluid content (TFC), assessed by thoracic electrical bioimpedance (TEB), in patients with CHF due to LVSD.
MethodsWe studied 120 patients with LVSD and CHF, referred to our laboratory for CPET: 76% male, age 52.1±12.1 years, 37% of ischemic etiology, left ventricular ejection fraction 27.6±7.9%, 83% in sinus rhythm, 96% receiving ACEIs and/or ARBs and 79% beta-blockers, and 20% treated with a cardiac resynchronization device. TEB studies were performed after 15 minutes of rest, prior to symptom-limited treadmill CPET, using the modified Bruce protocol. CPET-derived peak oxygen consumption (pVO2), the slope of the relationship between minute ventilation (VE) and carbon dioxide production (VCO2), VE/VCO2 at the anaerobic threshold (AT), and TFC assessed by TEB were considered for analysis.
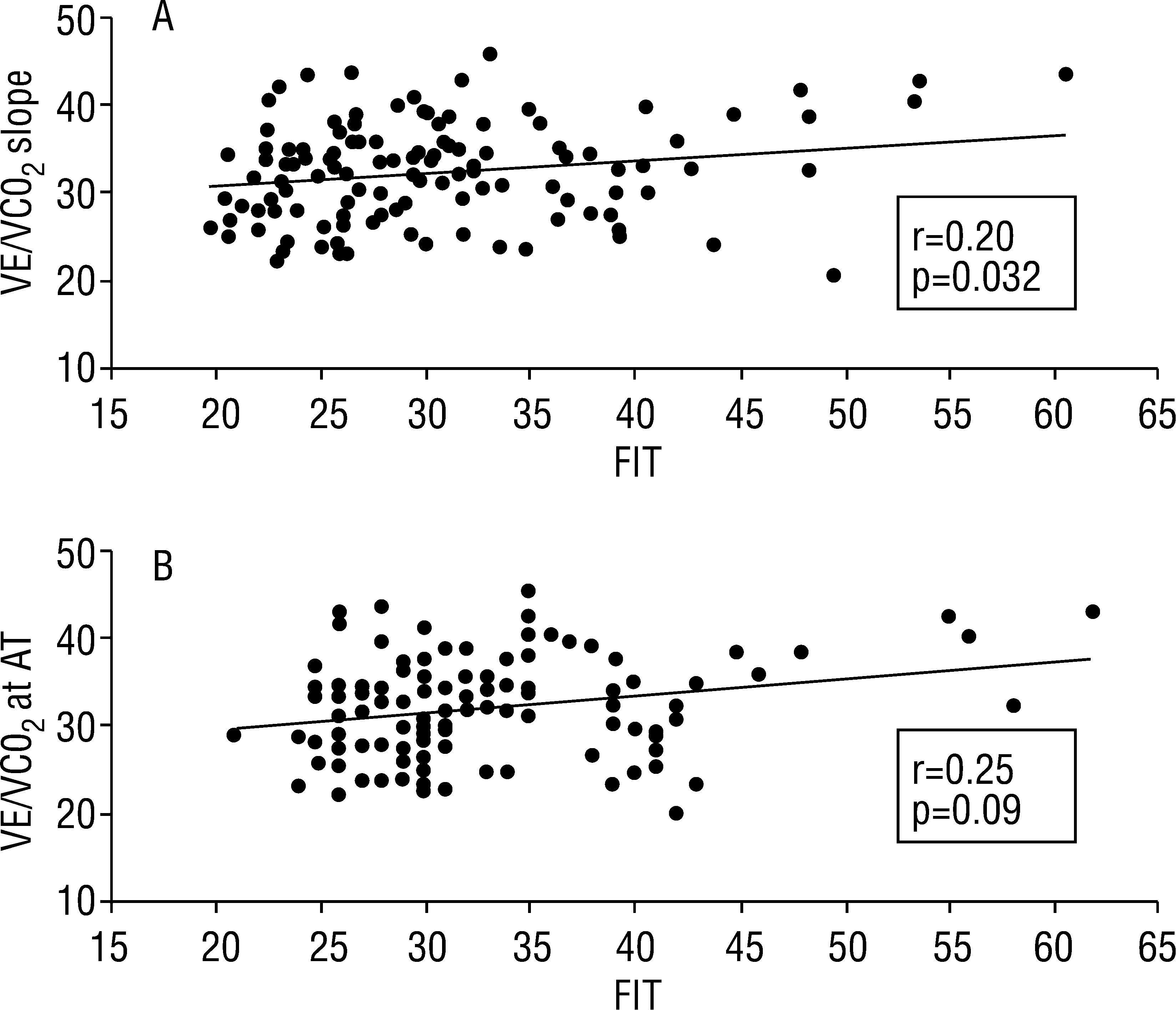
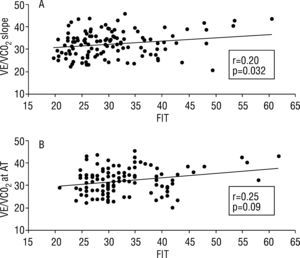
ResultsTFC ranged between 20.6 and 45.8kOhm−1, mean 32.2, SD=5.7, median 32.7, pVO2 8.9–40.6ml/kg/min, mean 21.0, SD 6.2, median 20.2, VE/VCO2 slope 19.8–60.7, mean 30.7, SD 7.9, median 29.1 and VE/VCO2 at AT 21–62, mean 33.1, SD 7.5, median 31.5. By linear regression, TFC did not correlate with pVO2 (r=0.05, p=0.58), but showed correlation with parameters of ventilatory efficiency: r=0.20, p=0.032, r2=0.04 for VE/VCO2 slope and r=0.25, p=0.009, r2=0.06 for VE/VCO2 at AT.
ConclusionTFC correlates with CPET parameters of ventilatory efficiency in patients with CHF due to LVSD, suggesting that it may be one of its determinants.
A eficácia ventilatória, avaliada por prova de esforço cardiorespiratória (PECR), tem um importante valor prognóstico em doentes (dts) com insuficiência cardíaca crónica (ICC) por disfunção sistólica ventricular esquerda (DSVE). Os seus determinantes mantêm-se, contudo, controversos.
ObjectivoInvestigar a eventual correlação entre parâmetros de eficácia ventilatória, obtidos por PECR, e o valor do fluido torácico total (FTT), avaliado por bioimpedância eléctrica torácica (BET), em dts com ICC por DSVE.
MétodosEstudámos 120 dts com ICC por DSVE, referenciados ao nosso laboratório para PECR - 76% do sexo masculino, idade 52,1 ± 12,1 anos, 37% de etiologia isquémica, fracção de ejecção ventricular esquerda 27,6 ± 7,9%, 83% em ritmo sinusal, 96% sob iECA e/ou ARAII, 79% sob beta-bloqueante e 20% tratados com dispositivo de ressincronização cardíaca. Os dts efectuaram PECR, em tapete rolante, protocolo de Bruce modificado, sendo considerados para análise, como parâmetro de capacidade funcional, o consumo de oxigénio de pico (VO2p) e, como parâmetros de eficácia ventilatória, o declive (d) da relação entre ventilação minuto (VE) e produção de CO2 (VCO2) e o valor do VE/VCO2 no limiar anaeóbico (LANA). Os estudos por BET, média de 20 minutos de aquisição, foram efectuados após 15 minutos de repouso, em posição supina, imediatamente antes das PECR, sendo analisado o valor do FTT.
ResultadosO valor do FTT variou entre 20,6 e 45,8kOhm−1, média=32,2, DP=5,7, mediana=32,7, o de VO2p entre 8,9 e 40,6ml/kg/min, média=21,0, DP=6,2, mediana=20,2, o do dVE/VCO2 entre 19,8 e 60,7, média=30,7, DP=7,9, mediana=29,1 e o do VE/VCO2 no LANA entre 21 e 62, média=33,1, DP=7,5, mediana=31,5. Por regressão linear, o FTT não se correlacionou com o VO2p - r=0,05, p=0,58 - mas apresentou correlação com os parâmetros de eficácia ventilatória analisados: r=0,20, p=0,032, r2=0,04 com dVE/VCO2 e r=0,25, p=0,009, r2=0.06 com VE/VCO2 no LANA.
ConclusãoO FTT correlaciona-se com os parâmetros de eficácia ventilatória, avaliados por PECR, em dts com ICC por DSVE, o que indica que poderá ser um dos seus determinantes.
Functional capacity and ventilatory efficiency assessed by cardiopulmonary exercise testing (CPET) are of established value in patients with chronic heart failure (CHF) due to left ventricular systolic dysfunction (LVDS)1,2. Functional capacity is generally determined by peak oxygen consumption (pVO2), while assessment of ventilatory efficiency is based on analysis of the relationship between minute ventilation (VE) and carbon dioxide (CO2) production - VE/VCO2 - or ventilatory equivalent for CO2, in practice the amount of air needed to eliminate one liter of CO2.
There are multiple determinants of functional capacity, particularly the interaction between pulmonary, cardiac and skeletal muscle function, as well as age, gender, muscle mass and conditioning status1. Despite this dependence on multiple factors, in fit individuals without pulmonary or skeletal muscle disease, pVO2 provides an indirect measure of cardiac output (CO), the correlation being determined by established formulas1,3,4.
Although the most important determinant of ventilatory efficiency is the correlation between ventilation and perfusion2, the mechanisms that lead to reduced efficiency in CHF patients remain the subject of debate5.
Thoracic electrical bioimpedance (TEB) was developed in the 1960s as part of NASA's space program6, as a non-invasive technique for continuous hemodynamic monitoring. It is based on the use of a high-frequency, low-amplitude electric current to calculate impedance of the flow of electricity through the chest. It measures instantaneous beat-by-beat changes in electrical impedance, on the basis of which algorithms have been developed to calculate parameters such as systolic volume, CO and thoracic fluid content (TFC), among others. The latest generation TEB systems are being used more widely following studies confirming their efficacy in various pathologies and correlation with invasive measurements7–9.
TFC, a new parameter assessed non-invasively by TEB, is an indicator of total chest fluid volume, both intra- and extracellular, particularly in the lungs10. In the absence of significant pleural and/or pericardial effusion, it can be used as an indirect measure of pulmonary perfusion.
ObjectiveThe aim of this study was to investigate the possible correlation between parameters of ventilatory efficiency obtained by CPET and TFC assessed by TEB, in patients with CHF due to LVSD.
MethodsWe studied 120 patients with LVSD and CHF, 91 (76%) male, 76 (63%) of non-ischemic and 44 (37%) of ischemic etiology, referred to our laboratory for a first CPET.
Only patients with left ventricular ejection fraction (LVEF) ≤40% on echocardiographic study in the previous 30 days were included; patients in New York Heart Association (NYHA) functional class IV and those with diseases that limited their ability to exercise or with moderate to severe lung, kidney or liver disease were also excluded. The presence of moderate or severe lung disease was assessed mainly through clinical history, observation and chest X-ray; only 24 patients (20%) underwent spirometry, which showed no significant alterations.
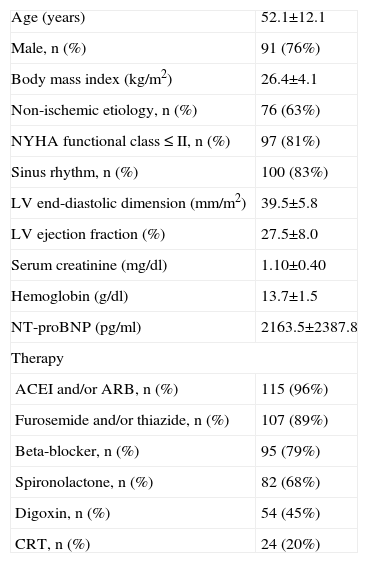
The patients' mean age was 52.1 ±12.1 years (21–77), body mass index 26.4±4.1 kg/m2 (17.6-37.6), left ventricular (LV) end-diastolic dimension 39.5±5.8 mm/m2 (31.9-57.0) and echocardiographic LVEF 27.5±8.0% (10-40%), and 83% were in sinus rhythm. According to the records of the physicians referring for CPET, 80.8% of the patients were in NYHA functional class ≤II. Mean serum creatinine was 1.10±0.40 mg/dl (0.6-2.0), hemoglobin 13.7±1.5 g/ dl (10.9-17.5), and NT-proBNP 2163.5±2387.8 pg/ml (54.8-10,924.0). With regard to medication, 96% were taking angiotensin-converting enzyme inhibitors and/ or angiotensin receptor blockers, 89% furosemide and/ or thiazide, 79% beta-blockers, 68% spironolactone, and 45% digoxin, and 20% were treated with a cardiac resynchronization device. The characteristics of the study population are summarized in Table.
Characteristics of the study population (n=120)
| Age (years) | 52.1±12.1 |
| Male, n (%) | 91 (76%) |
| Body mass index (kg/m2) | 26.4±4.1 |
| Non-ischemic etiology, n (%) | 76 (63%) |
| NYHA functional class ≤II, n (%) | 97 (81%) |
| Sinus rhythm, n (%) | 100 (83%) |
| LV end-diastolic dimension (mm/m2) | 39.5±5.8 |
| LV ejection fraction (%) | 27.5±8.0 |
| Serum creatinine (mg/dl) | 1.10±0.40 |
| Hemoglobin (g/dl) | 13.7±1.5 |
| NT-proBNP (pg/ml) | 2163.5±2387.8 |
| Therapy | |
| ACEI and/or ARB, n (%) | 115 (96%) |
| Furosemide and/or thiazide, n (%) | 107 (89%) |
| Beta-blocker, n (%) | 95 (79%) |
| Spironolactone, n (%) | 82 (68%) |
| Digoxin, n (%) | 54 (45%) |
| CRT, n (%) | 24 (20%) |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CRT: cardiac resynchronization therapy; LV: left ventricular; NYHA: New York Heart Association.
Patients underwent symptom-limited maximal CPET on a computerized treadmill using the modified Bruce protocol. A SensorMedics Vmax 229 system (Yorba Linda, Calif.) was used for analysis of respiratory gases and continuous 12-lead electrocardiographic monitoring. The equipment was calibrated before each test. Collection and analysis of respiratory gases was begun three minutes before exercise with the patient in a standing position and was continued until the sixth minute of recovery, as was ECG monitoring. Blood pressure was measured at rest, before each increment, at peak exercise, and at the first, third and sixth minute of recovery.
VO2, VCO2 and VE were assessed breath-by-breath, with pVO2 calculated as the mean of the last 30 seconds of the test. The VE/VCO2 slope was calculated by the system's computer.
The patients were encouraged to continue until the VCO2/ VO2 ratio (respiratory quotient) was ≥1.09. The anaerobic threshold (AT), determined using the V-slope method and corrected when necessary using the ventilatory equivalent for O2, was achieved by all patients.
In no case did blood pressure changes, arrhythmia, angina or ECG alterations prompt interruption of the test in accordance with the criteria stipulated in international guidelines11, and thus all exams were terminated due to fatigue or dyspnea as perceived by the patient.
The patients' usual medication was not suspended before the test.
We analyzed pVO2 (ml/kg/min) and, as ventilatory efficiency parameters, VE/VCO2 slope and VE/VCO2 at AT.
TEB methodologyTEB studies (BioZ® ICG Monitor, CardioDynamics) were performed immediately prior to CPET, with the patient in dorsal decubitus and the sensors placed on the neck and lateral chest wall. The mean acquisition time was 20 minutes.
In the present study, the value of TFC (kOhm-1) was considered for the analysis.
No patient presented pleural or pericardial effusion (as assessed by clinical history, chest X-ray and echocardiography), which would influence assessment of TFC, or more than mild aortic regurgitation, which would invalídate assessment by TEB.
Statistical analysisThe results for the variables under analysis are presented as means ±1 standard deviation (SD) and medians.
Correlations between variables were tested using Pearson's correlation coefficient since they presented a normal distribution. A value of p <0.05 was considered statistically significant. The coefficient of determination (r2) was calculated for the results obtained.
Receiver operating characteristic (ROC) curves were constructed based on the logistic regression models to assess the quality of the correlations by calculating the area under the curve (AUC) and to determine the TFC cut-off for the CPET parameters analyzed.
ResultsTFC ranged between 20.6 and 45.8 kOhm-1, mean 32.2, SD 5.7, median 32.7. The values for the CPET parameters analyzed were: pVO2 8.9-40.6 ml/kg/min, mean 21.0, SD 6.2, median 20.2; VE/VCO2 slope 19.8-60.7, mean 30.7, SD 7.9, median 29.1; and VE/VCO2 at AT 21–62, mean 33.1, SD 7.5, median 31.5.
By linear regression, there was a strong correlation between CPET parameters: pVO2 and VE/VCO2 slope - r=0.64, p <0.001; pVO2 and VE/VCO2 at AT - r=0.66, p <0.001; and VE/VCO2 slope and VE/VCO2 at AT - r=0.88, p <0.001.
TFC did not correlate with pVO2 (r=0.05, p=0.58), but showed correlation with parameters of ventilatory efficiency: r=0.20, p=0.032, r2=0.04 for VE/VCO2 slope and r=0.25, p=0.009, r2=0.06 for VE/VCO2 at AT (Figure 1).
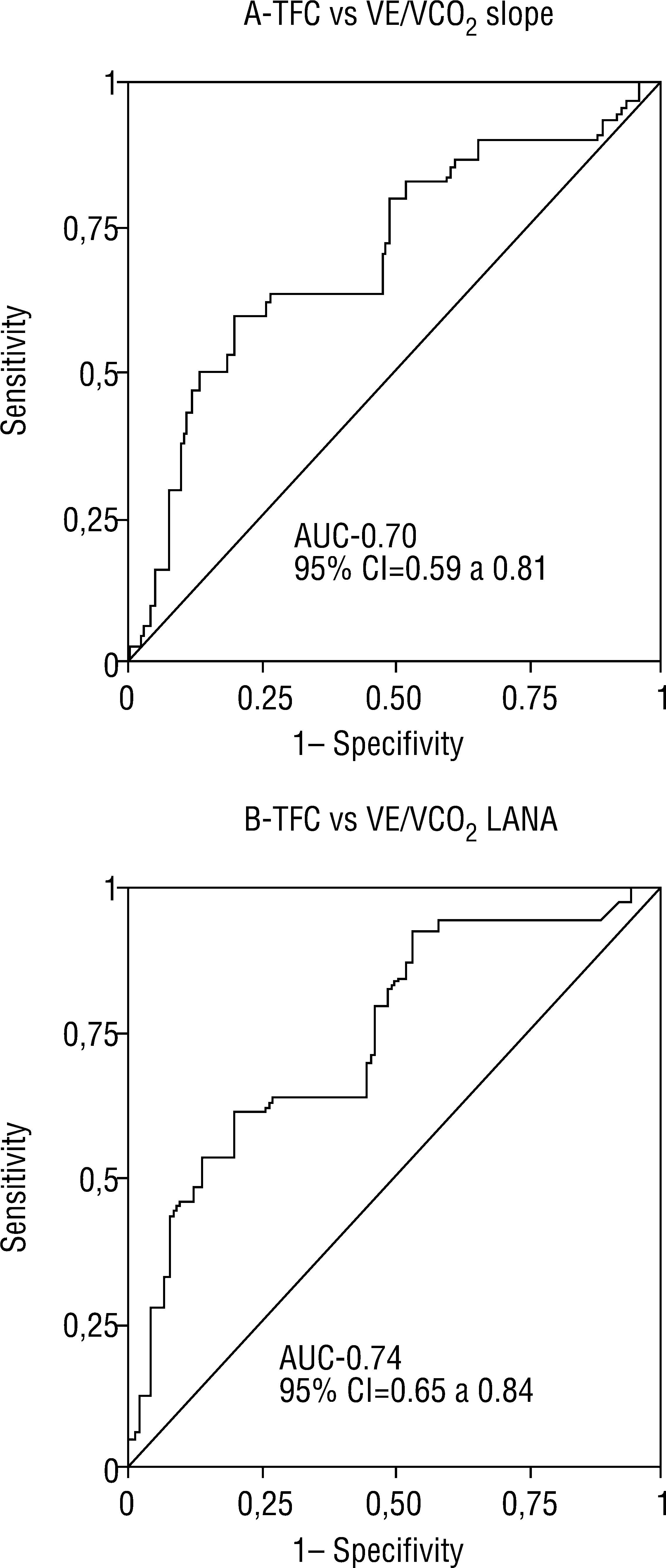
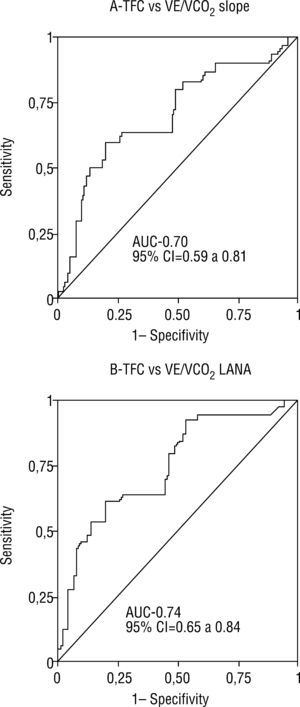
ROC curves were used to assess the quality of the correlations between TFC determined by TEB and ventilatory parameters by CPET. For VE/VCO2 slope ≥34, the AUC was 0.70, with 95% confidence interval (CI) of 0.59-0.81 (Figure 2). TFC >35.9 had sensitivity of 60% and specificity of 80% for VE/VCO2 ≥34. For VE/VCO2 at AT ≥34, the AUC was 0.74, with 95% CI of 0.65-0.84 (Figure 2). TFC >34.9 had sensitivity of 65% and specificity of 84% for VE/VCO2 at AT ≥34.
DiscussionThe present study found a correlation, albeit of low statistical significance, between resting TFC and CPET parameters assessing ventilatory efficiency. TFC can be used as an indirect measure of pulmonary perfusion; a previous study by our group established a significant correlation between TFC and pulmonary capillary wedge pressure (PCWP)9. These results are in agreement with those of Reindl et al.12, who demonstrated a positive correlation between resting PCWP and pulmonary artery pressure and VE/VCO2 slope.
Lewis et al.5 also found a significant correlation between resting PCWP and VE/VCO2 slope. Chronically elevated left ventricular filling pressures may contribute to inadequate pulmonary vasodilation and a high dead space-to-tidal volume ratio (VD/VT) during exercise through pulmonary vascular remodeling. On the other hand, no correlation was seen between VE/VCO2 slope and PCWP during exercise, suggesting that ventilatory efficiency is not dictated simply by acute changes in left ventricular filling pressures during exercise; for these authors, changes in pulmonary vasomotor tone and right ventricular function may also be important determinants of ventilatory efficiency in patients with CHF.
The role of muscle ergoreceptor overactivity in hyperventilation during exercise in CHF patients has also been investigated13–15. The ergoreflex, which is heightened in these patients, is a complex metabolic reflex in which peripheral chemoreceptors in muscle react to local metabolic byproducts during exercise and initiate a neural reflex that drives hyperventilation5. Although the relative contribution of the ergoreflex to this phenomenon remains to be fully clarified, Guazzi et al.16 have demonstrated that long-term use of sildenafil in CHF patients attenuates this reflex and improves VE/VCO2 slope.
To summarize, many different factors determine ventilatory efficiency in patients with CHF. The present study demonstrated that it correlates with TFC as assessed by TEB, and we postulate that the value of this new parameter is not restricted to its correlation with PCWP. TFC, a measure of the total volume of chest fluids, both extra- and intracellular, provides better and more reliable assessment of changes than traditional hemodynamic parameters10. Okawa et al., in a study of patients with acute myocardial infarction, also found a significant correlation between TFC and PCWP, which was independent of cardiac index17. However, their results suggest that TFC is a better predictor of the degree of pulmonary congestion, particularly when PCWP is <18 mmHg.
Our aim was to investigate the possible correlation between TFC assessed by TEB and parameters of ventilatory efficiency determined by CPET. However, we also analyzed pVO2, and in the TEB studies performed prior to CPET, cardiac output was also determined. While as expected, pVO2 presented a strong correlation with ventilatory efficiency parameters, no significant correlation was found with TFC, suggesting that this is not a determinant of pVO2. Resting CO assessed by TEB showed a mean value of 4.5±1.2 l/min (1.9-8.1). No significant correlation was found between CO and TFC (r=0.156, p=0.10), nor with VE/ VCO2 slope (r=0.169, p=0.07) or VE/VCO2 at AT (r=0.118, p=0.23), the correlation being significant only with pVO2 (r=0.208, p=0.023). While not within the scope of the present study, these results support the idea that CO, even assessed at rest, is a determinant of pVO21,3,4.
Most studies investigating correlations between hemodynamic parameters, obtained either invasively or non-invasively, and functional capacity and ventilatory efficiency assessed by CPET, have been based on analysis of hemodynamic parameters at rest. Interestingly, when these parameters are assessed during exercise, either they do not correlate with CPET variables (with the exception of pVO2 and CO)5, or they provide no additional prognostic value1. This surprising finding may be explained by the fact that there are multiple determinants of CPET parameters, including cardiac reserve and skeletal muscle, pulmonary and endothelial (dys)function. The correlation between ventilatory efficiency determined by VE/VCO2 slope and hemodynamic variables assessed at rest would appear less controversial, since it is constant at submaximal exercise.
The main limitations of the present study are that spirometry was not performed and that changes in TFC during exercise were not assessed.
ConclusionThoracic fluid content determined by thoracic electrical bioimpedance correlates with CPET parameters of ventilatory efficiency in patients with congestive heart failure due to left ventricular systolic dysfunction, suggesting that it may be one of its determinants.
Conflicts of interestThe authors have no conflicts of interest to declare.