Transcatheter aortic valve implantation is an emerging treatment option for severe symptomatic aortic stenosis in patients considered unsuitable for surgical valve replacement. The authors review the use of multislice computed tomography in the selection of candidates for transcatheter aortic valve replacement, procedural support and post-interventional follow-up. A single-center experience of the role of this imaging technique is also described.
Multislice computed tomography is an essential imaging tool in the selection and exclusion of candidates for transcatheter aortic valve implantation, providing evaluation of coronary anatomy and the relationship of the coronary ostia with the aortic valve structure, and accurate analysis of the valve annulus and aortic root, left ventricular outflow tract, aorta and peripheral vascular access routes. Multislice computed tomography is also central to the choice of appropriate prosthesis size. In addition, it guides arterial puncture by image fusion techniques and enables correct prosthesis apposition to be verified. This review aims to describe the role of computed tomography in this increasingly common interventional valve procedure, providing an overview of current knowledge and applications.
A substituição valvular aórtica percutânea é uma opção emergente para o tratamento da estenose aórtica grave sintomática em doentes recusados para substituição valvular cirúrgica. Os autores fazem uma revisão da literatura na utilização da Tomografia Computorizada Multicorte na avaliação de candidatos a implantação de prótese aórtica percutânea, no apoio ao procedimento e seguimento pós-intervenção. Os autores descrevem, ainda, a experiência de um Centro na utilização desta técnica de imagem no contexto de substituição valvular aórtica percutânea.
A Tomografia Computorizada Multicorte é um método de imagem de eleição na selecção e exclusão de candidatos a implantação valvular aórtica percutânea, permitindo avaliação da anatomia coronária e relação dos ostia coronários com a estrutura valvular aórtica, avaliação precisa do anel valvular e restante raiz aórtica, câmara de saída do ventrículo esquerdo, aorta e acessos vasculares periféricos. As imagens obtidas por Tomografia Computorizada Multicorte são informação central na escolha da dimensão da prótese a implantar, permitem apoio à punção vascular durante o procedimento por métodos de fusão de imagem e efectuar seguimento à adequada aposição da prótese.
Degenerative aortic valve disease currently accounts for most cases of native valve disease and is a cause of significant morbidity and mortality, especially in the elderly1. Aortic stenosis progresses slowly long before the appearance of symptoms, after which mortality in the first two years is over 50%2–4. Simple valvotomy does not alter the natural history of aortic stenosis5, and valve replacement is the only effective treatment in advanced stages of the disease. Surgery has good results in most patients, and with careful selection of candidates, prosthetic valves have good durability, even in the elderly1,6–10. However, a significant proportion (30-40%) of patients with severe aortic stenosis are not referred or are considered unsuitable for valve replacement surgery2,5, usually because of advanced age, left ventricular systolic dysfunction, or multiple comorbidities5,6,11.
Since the first percutaneous implantation of an aortic valve in a human (by Alain Cribier12 in 2002), transcatheter aortic valve implantation (TAVI) has undergone significant advances and is now a viable treatment option for high-risk groups4. The number of centers worldwide with experience in this technique has grown significantly and the number of procedures has risen exponentially; over 50,000 high-risk patients have undergone TAVI, with a success rate of around 95% and 30-day mortality of 5-18%13.
Despite the evidence of good short- and medium-term results, there are safety issues with the TAVI technique, including vascular complications, optimization of delivery and positioning of the prosthesis, the long-term consequences of paravalvular leak, ischemic vascular events, atrioventricular block requiring temporary or permanent ventricular pacing, and the durability of the prosthesis itself14. Two percutaneous prosthetic valves are currently approved in Europe: the balloon-expandable Edwards SAPIEN (Edwards Lifesciences Inc, Irvine, California, USA), and the self-expanding CoreValve (Medtronic Inc, Minneapolis, Minnesota, USA).
Imaging techniques are central to patient selection and preparations. Unlike surgery, in which there is direct anatomical exposure, in transcatheter implantation the operator must rely on pre- and intraprocedural imaging techniques capable of acquiring large quantities of information that is then processed for multiplanar or three-dimensional reconstructions15.
Transthoracic and transesophageal echocardiography and contrast aortography have been the most frequently used imaging methods in recent years to determine the size of the aortic annulus and root, as well as assisting in the selection of an appropriate prosthesis15. Advances in computed tomography (CT), including an increasing number of detectors and hence better spatial and temporal resolution than previous generations, means multislice CT (MSCT) can provide detailed anatomical information on the aortic annulus, the anatomic relationship of the annulus to the coronary ostia, and the aortic arch13,15. In selected patients, MSCT also provides valuable pre-interventional information on arterial access for delivery of the valve prosthesis. It thus contributes in various ways to the diagnosis and management of severe aortic stenosis.
This article reviews the contribution of MSCT in preprocedural assessment prior to TAVI, with particular reference to the CoreValve prosthesis, as this is the device used in our center.
The CoreValve aortic prosthesisThe CoreValve is a trileaflet porcine pericardial tissue bioprosthesis, which is mounted and sutured in a self-expanding nitinol stent. Two sizes are available, 23 mm (for 20–24 mm aortic annulus) and 29 mm (for 24–27 mm aortic annulus). The delivery system, currently in its third generation, has an 18F sheath15.
The valve is transported in an introducer sheath via a retrograde transfemoral or subclavian approach and expands to its predefined shape when the sheath is withdrawn. It consists of three parts: the lower portion has high radial force to expand and prevent collapse of the calcified leaflets; the middle portion includes the porcine pericardial tissue and is constrained in order to avoid occluding the coronary arteries; and the upper portion fixes the prosthesis in the ascending aorta15. Delivery takes place under rapid ventricular pacing. Post-dilation can be performed if necessary, depending on prosthesis position and the presence of aortic regurgitation.
Selection of patients for transcatheter aortic valve implantationSelection of patients for TAVI is influenced by a variety of considerations. Despite recent advances in techniques, clinical assessment is essential in the management of patients with severe aortic stenosis, in the selection of candidates for TAVI and in decisions on appropriate timing and procedure, taking into consideration current evidence and the patient's wishes and quality of life. Essential to this process is quantification of the degree of stenosis, for which echocardiography is the first-line method due to its availability and reliability16.
Evaluation of surgical risk combines clinical assessment and a range of validated risk scores (EuroSCORE, STS Predicted Risk of Mortality, Ambler Risk Score), of varying precision17. TAVI is not recommended if life expectancy is less than one year16.
Patient selection for TAVI involves four steps: 1) confirmation of the severity of aortic stenosis; 2) evaluation of symptoms; 3) analysis of surgical risk, life expectancy and quality of life; and 4) assessment of the feasibility of the procedure and exclusion of contraindications16. The first three are beyond the scope of this document.
The decision on a patient's suitability for TAVI should involve a team of cardiologists, cardiac surgeons and anesthesiologists, as well as the candidate's physician16. Selection is based on international guidelines and the recommendations of the manufacturer of the percutaneous device. Table 1 summarizes the recommendations for selecting candidates for TAVI using the CoreValve device, including imaging modalities for each criterion. The latest recommendations include greater emphasis on use of MSCT and magnetic resonance imaging (MRI). In our opinion, as well as in that of other authors18–21, MSCT provides additional information, such as identifying the presence of atrial or ventricular thrombi and estimating left ventricular ejection fraction if the entire cardiac cycle is acquired, data usually determined by echocardiography.
Indications and general guidance for selection of candidates for transcatheter implantation of a CoreValve device and for selection of prosthesis size (adapted from the manufacturer's recommendations)
*: elements that are not among the manufacturer's recommendations but can be determined by MSCT. Source: Medtronic Inc., USA.
Ao: aortic; CAG: coronary artery angiography; CT: computed tomography; MRI: magnetic resonance imaging.
TAVI with the CoreValve device is not recommended in the following situations:
- –
Presence of intraventricular thrombus or subaortic stenosis
- –
Vascular access <6 mm in caliber
- –
Left ventricular ejection fraction <20% with no contractile reserve
- –
Aortic annulus <20 or >27 mm
- –
Bicuspid aortic valve
- –
Significant asymmetric valvular calcification
- –
Aortic root diameter >43 mm
- –
Severe vascular disease in general
Multiple imaging modalities are particularly useful for the evaluation of patients with cardiovascular disease and in vascular procedures. MSCT has a central role in the planning of TAVI.
Planning TAVICurrent recommendations16 identify various parameters that should be taken into consideration when planning TAVI. In general, the following should be assessed:
1Assessment of coronary anatomyConventional coronary angiography is recommended to assess the coronary anatomy16, although non-invasive assessment can also be performed by MSCT, with good results in individuals with valve disease, even in the presence of irregular cardiac rhythms22. It also has the advantage of accurately determining the location of the coronary ostia and their relationship with the valve leaflets23–25. This information is important because variations in anatomy can affect the feasibility of the procedure26. However, in the patients currently referred for TAVI, multislice CT angiography is of limited value, since the high prevalence of coronary atherosclerosis and severe coronary calcification in this age-group greatly reduces the technique's diagnostic accuracy in excluding coronary disease. In the future, widening the application of TAVI to younger populations with fewer comorbidities may increase the value of CT angiography in this context, but at present the diagnosis of coronary disease requires invasive coronary angiography.
If coronary disease requires revascularization, whether to proceed surgically, percutaneously, or in a hybrid manner, as well as the timing of the intervention, should be the subject of discussion by the multidisciplinary team. TAVI is not recommended in patients with proximal coronary stenoses not amenable to revascularization.
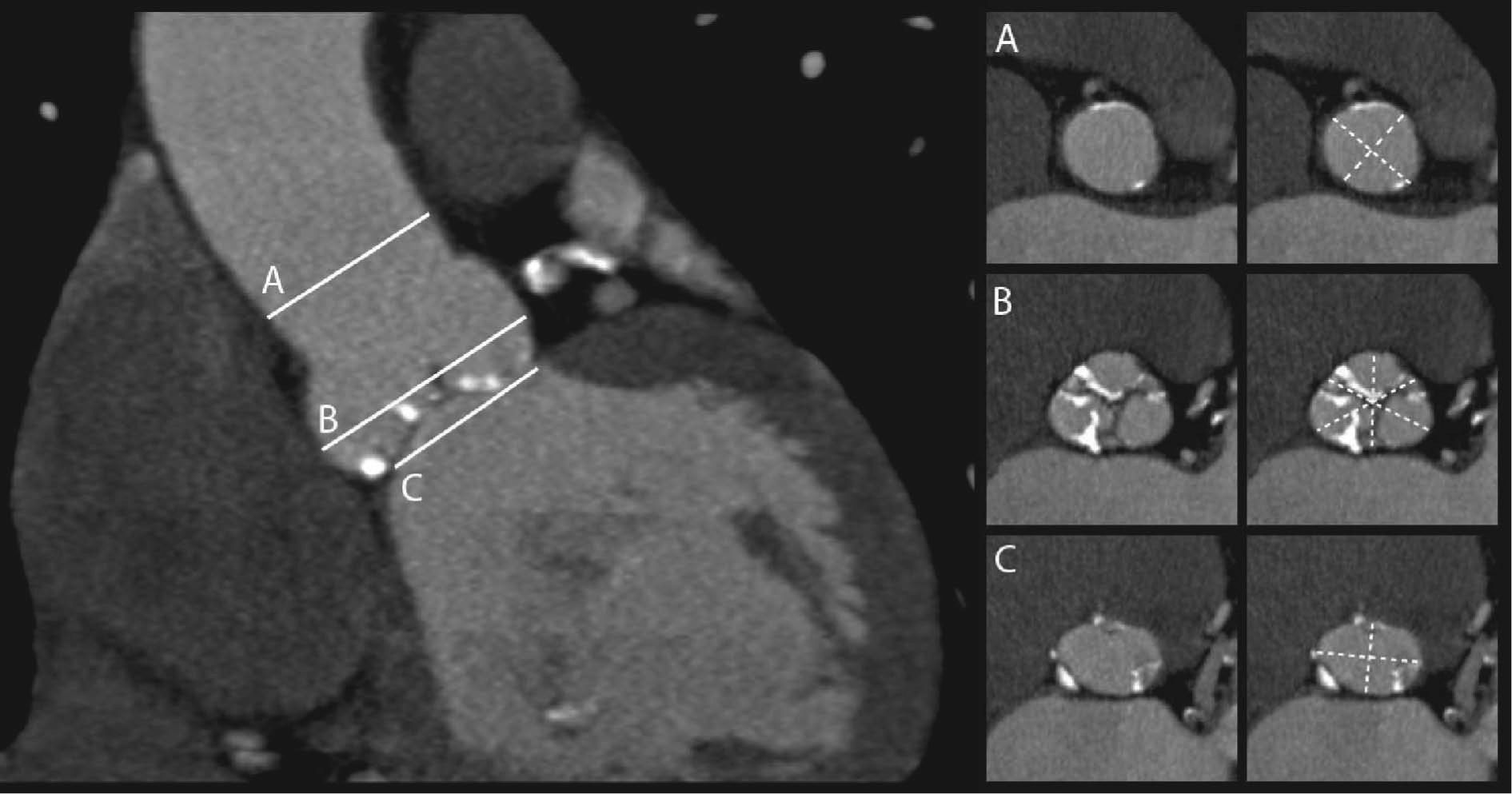
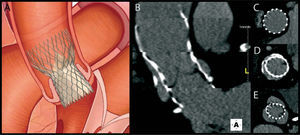
2Measurement of the aortic annulusThe dimensions of the aortic annulus determine prosthesis size, hence accurate measurement of this parameter is critical in assessing candidates for TAVI to minimize the potential for paravalvular leak and to avoid prosthesis migration. Although a gold standard method of measurement has yet to be established, the joint guidelines of the European Association of Cardio-Thoracic Surgery, the European Society of Cardiology and the European Association of Percutaneous Cardiovascular Interventions state that MSCT and MRI can provide a more accurate assessment of the valve annulus and adjoining structures than two-dimensional transthoracic or transesophageal echocardiography27 (Figure 1.).
The aortic annulus has a complex three-dimensional structure that is not in a single plane and is not circular but crown-shaped. For sizing purposes, it should be represented as a virtual ring defined by the caudal tangent of the three anchor points of the valve leaflets that is elliptical in shape26–31. It is therefore not strictly a ring.
In surgical valve repair, non-invasive estimates by the usual methods of transthoracic and transesophageal echocardiography are not entirely reliable for selecting the correct prosthesis size, and dilators are used during the procedure to measure the aortic annulus. Additionally, in surgical replacement accuracy of measurement may be of less importance, since the prosthesis is sewn to adjacent tissues28. In TAVI, measurements are made solely by imaging techniques, which are therefore of greater importance. A study of 33 patients undergoing surgical aortic valve replacement showed good agreement between aortic annulus dimensions measured by CT and by direct intraoperative methods32. Measurements obtained by MSCT present less inter-observer variability than by transthoracic echocardiography and contrast aortography, but not by transesophageal echocardiography33,34.
3Assessment of the aortic root and ascending aortaAs for the valve and annulus, the anatomy of the aortic root and ascending aorta are important elements in patient selection, prosthesis sizing and anticipation of possible intraprocedural complications.
Conventional echocardiography, being two-dimensional, is of little use in assessing the aortic root30, whereas MSCT provides precise three-dimensional anatomical data. Measurement of the diameter of the sinuses of Valsalva and the sinotubular junction (although the latter is no longer mentioned in the latest version of the manufacturer's recommendations) is important for prosthesis sizing, and the accuracy of these measurements will influence the incidence of paravalvular leak after placement35.
Assessment of the relationship between the aortic leaflets and coronary ostia is important to avoid occlusion of the coronary arteries during apposition of the prosthesis36. The anatomical relationship between the aortic annulus, coronary ostia and valve leaflets is known to be highly variable. Of a cohort of 169 patients, in 49% the distance between the annulus and the ostia was less than the length of the leaflets35. This increases the risk of ostial occlusion during the procedure, although this can be resolved in some cases by angioplasty or bypass surgery37. Finally, it is necessary to exclude severe angulation of the ascending aorta, which may be a contraindication, and to obtain adequate anatomical information to ensure correct apposition of the upper part of the valve prosthesis to the wall of the aorta.
The procedure is not recommended if the height and/or diameter of the sinuses of Valsalva are less than 15 mm and 29 mm, respectively, or if the diameter of the ascending aorta is over 43 mm.
4Assessment of the left ventricular outflow tractThe left ventricular outflow tract consists of a larger muscular component and a fibrous component. Significant subaortic protrusion or septal hypertrophy may hamper correct apposition of the prosthesis and are thus considered a contraindication to TAVI by some authors26.
5Assessment of peripheral vascular accessThe prevalence of peripheral arterial disease is high among candidates for TAVI. Despite technical advances that have led to lower profile (smaller diameter) delivery systems, the presence of severe calcification (particularly circular/circumferential), arterial tortuosity, small lumen diameter (generally <6-9 mm, depending on the delivery system), and significant stenosis are associated with potentially fatal vascular access complications, and these situations must therefore be excluded in the preprocedural study38. MSCT provides assessment with millimeter resolution of the entire course of the vessel up to the aortic root, whether via the femoral or subclavian route, and is the method of choice for this purpose38.
Other parameters that can be measured by MSCT in the assessment of candidatesMSCT additionally enables evaluation of other parameters that are important in the assessment and stratification of aortic stenosis:
A. Patient selection: measurement of aortic valve area by planimetry. Various methods can be used to measure valve area, each based on different physical principles. With echocardiography, the current standard technique39,40, the area is estimated by the continuity equation using Doppler flows. The anatomical area can also be estimated by valve planimetry, although the spatial resolution is low. Left heart catheterization is used to estimate valve area on the basis of gradients and cardiac output. MSCT visualizes the valve structure directly with sufficient resolution to perform planimetry. As with echocardiography, it is important to select the correct planes to accurately measure the orifice, which is defined by the tips of the valve leaflets; in some cases the orifice is not in a parallel plane to the annulus, which can result in underestimation of the area38.
In general, imaging modalities that use hemodynamic methods to estimate valve area differ systematically from those that use anatomical parameters. Although it tends to underestimate the area, mainly due to the assumption that the left ventricular outflow tract is uniformly cylindrical, echocardiography remains the method of choice, because of its accessibility, good results, and non-invasive nature. Measurement by MSCT planimetry correlates well with transesophageal echocardiography and can be used as an alternative if other methods fail or produce contradictory results, notwithstanding its tendency to overestimate valve area38,41,42. Halpern et al. showed that when the left ventricular outflow tract area measured by CT angiography was used in the continuity equation, the differences between 64-slice MSCT and echocardiography decreased from 0.60 to 0.14 cm243. This adjustment is particularly useful in severe aortic stenosis44.
B. Assessment of aortic valve anatomy. The precise anatomy and morphology of the aortic valve must be carefully determined, since they have important clinical and technical implications. Although this is conventionally performed by echocardiography, MSCT provides a highly reliable assessment.
The presence of bicuspid aortic valve is associated with higher rates of prosthesis malpositioning and of paraprosthetic regurgitation, and thus TAVI is not recommended in such cases45. Investigation of the location and extent of valve calcification can aid in anticipating procedure-related complications such as malapposition of the prosthesis to the valve annulus and aortic root, leading to regurgitation, calcium embolization and increased difficulty in passing the catheter through the valve orifice13. MSCT can determine valve morphology and is the method of choice for identifying and accurately localizing areas of valvular and arterial calcification.
c. Assessment of valvular calcification. In most cases, severe aortic stenosis is the result of a degenerative process that includes calcification. Rosenhek et al.46 demonstrated that the degree of valve calcification predicts prognosis in severe aortic stenosis, being worse in patients with moderate to severe calcification, and suggested early valve replacement in such patients. The degree of calcification can only be determined approximately by echocardiography, whereas MSCT allows accurate detection and quantification of aortic valve calcification with high reproducibility38. Pohle et al. found an association between the number of cardiovascular risk factors and aortic valve calcification in a population of 1000 patients undergoing CT study47.
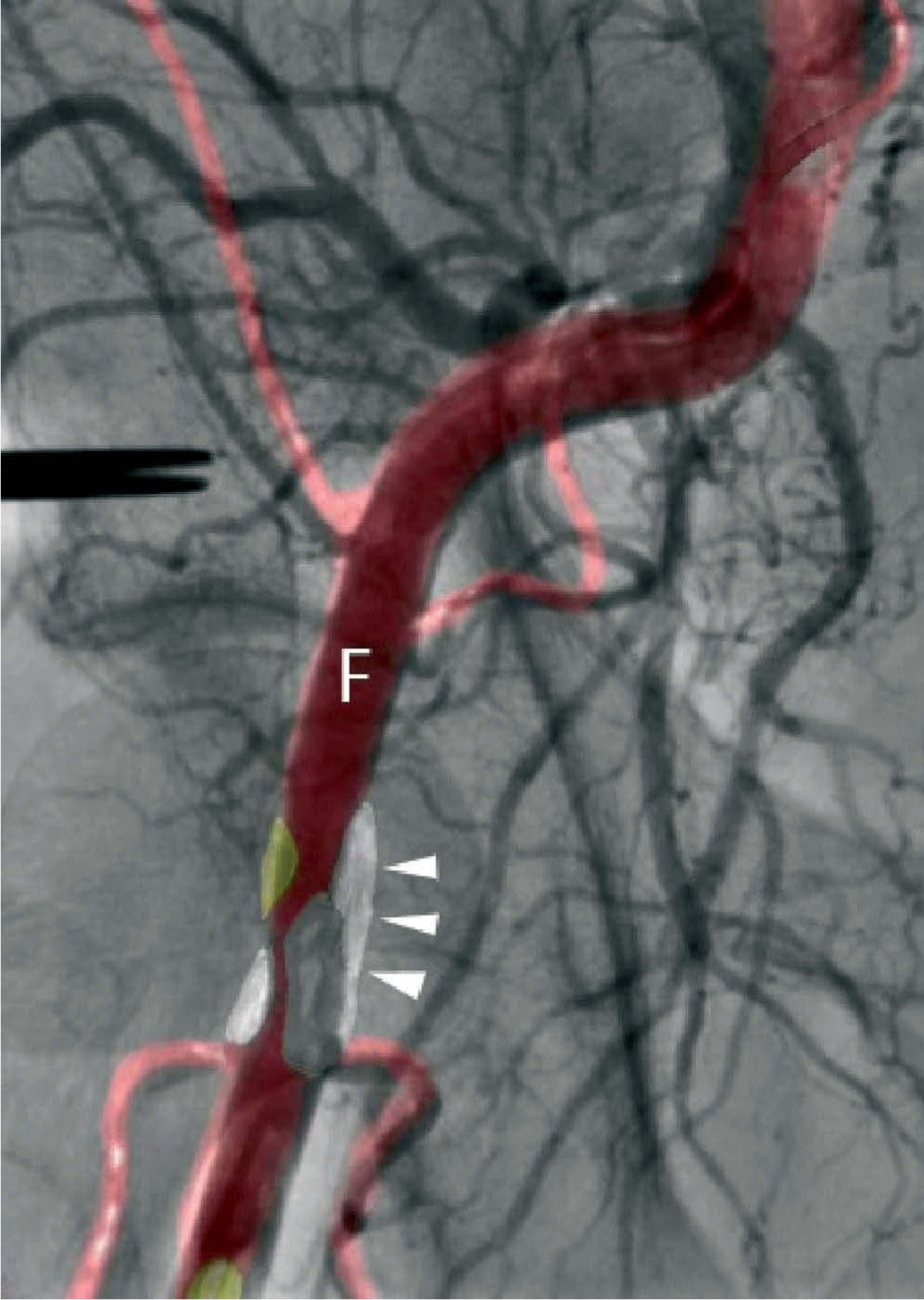
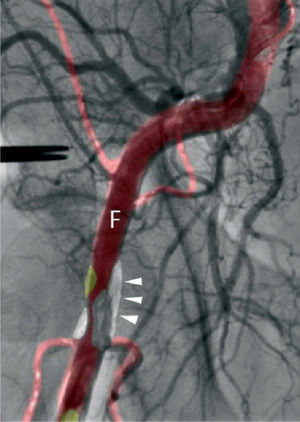
Procedural supportPercutaneous valve replacement systems have undergone various improvements to increase the ease and precision of device guidance and release. Nevertheless, bleeding at the access site is a major cause of mortality and morbidity in patients undergoing TAVI. With the development of fusion imaging, combining CT with angiography, it is now possible to determine the position of atheromatous plaques that cannot be visualized by conventional angiography, thus enabling the operator to select the most suitable puncture site and needle alignment in order to minimize the risk of complications due to plaque perforation (Figure 2.). Recent studies have shown good results in identifying plaques, assessing and managing bifurcations, and reconstructing planes that cannot be visualized by angiography. However, further studies are needed to validate the clinical usefulness of fusion imaging and its impact on reduction of radiation exposure30.
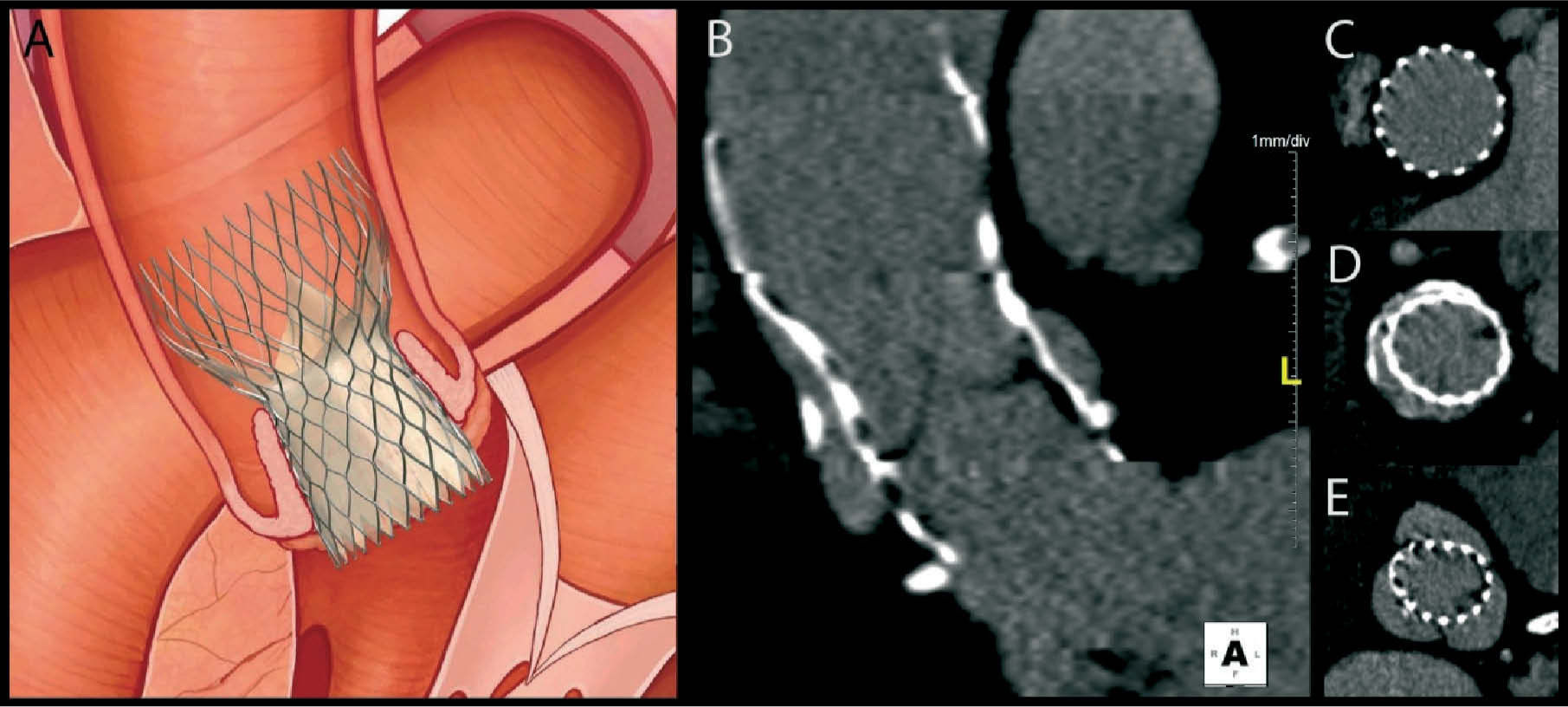
Post-interventional follow-upFollow-up of patients who have undergone TAVI is essentially clinical. Assessment of the function and position of the prosthesis will confirm the success of the procedure and the patient's prognosis. Echocardiography, which is readily accessible and less invasive, is as essential for this purpose as for the initial evaluation, providing structural and Doppler flow information. Postprocedural MSCT, with its high spatial resolution, adds information on the correct positioning and expansion of the prosthesis and excludes complications (Figure 3.) related to the implantation procedure or to the vascular access site48.
Experience with postprocedural MSCT indicates that incomplete and nonuniform expansion of the nitinol frame of the CoreValve is common in the proximal and distal segments, whereas the mid segment — the functional segment and hence the most important — expands correctly and almost symmetrically48. This is probably related to the pressure exerted by the calcified native leaflets and to the final position of the prosthesis in the aortic root. Surprisingly, Schultz et al.48 found undersizing of the valve prosthesis in most of the study population, although this was small. There is as yet no evidence as to whether this finding has practical implications.
Disadvantages of MSCTDespite the various advantages of MSCT, it should be borne in mind that obtaining the images requires injection of contrast medium in patients who frequently have some degree of renal dysfunction, and also involves varying doses of ionizing radiation. These disadvantages may be reduced as acquisition techniques improve and with new CT systems47,49,50. Furthermore, ejection volumes, flows and transvalvular gradients cannot be determined by MSCT in isolation.
Experience of the Department of Cardiology of Vila Nova de Gaia/ Espinho Hospital CenterThe Department of Cardiology of Vila Nova de Gaia/ Espinho Hospital Center was the first center in the Iberian Peninsula to perform transcatheter aortic valve implantations, in August 200751. The CT equipment used is a Somatom Sensation Cardiac 64 (Siemens AG), which is in daily use for studies and procedures in the Department of Cardiology and for studies in other departments. It is an essential tool in the evaluation of candidates for TAVI.
Up to March 2011, 136 candidates for TAVI had been evaluated by MSCT. Of these, 44% were male and mean age was 78.3±9.2 years (Table 2).
Characteristics of the study population
| Female | Male | Total | |
| Total (n.%) | 76 (55.9) | 60 (44.1) | 136 (100) |
| Age (years) | 80.6±6.2 | 75.3±11.4 | 78.3±9.2 |
| Maximum annulus diameter (mean, mm) | 25.2±3.1 | 24.4±3.4 | 24.9±3.3 |
| Minimum annulus diameter (mean, mm) | 18.7±3.2 | 19.1 ±3.1 | 18.9±3.1 |
| Sinus of Valsalva height (mm) | 19.2±3.6 | 18.8±3.8 | 19.0±3.7 |
| Sinus of Valsalva width (mean, mm) | 27.4±3.1 | 32.1±4.0 | 29.3±4.2 |
| Maximum ascending aorta diameter (mm) | 35.7±7.0 | 35.7±4.8 | 35.8±8.3 |
| Minimum ascending aorta diameter (mm) | 35.6±4.8 | 33.5±4.8 | 33.3±7.2 |
Image acquisition is performed in two stages: a non-synchronized scan of the vascular tree from the femoral branches to the neck vessels, and a second ECG-gated (synchronized) scan for optimized visualization of the aortic root and annulus and the coronary ostia. In individuals with significant renal dysfunction (creatine clearance <30 ml min—1), only the first stage is performed. The mean radiation dose in the synchronized and non-synchronized scans was 433.9±134.9 and 524±172.5 mGy.cm, respectively. Although the additional synchronized scan increases the radiation dose, we consider that the information obtained by precise assessment of the aortic root, sinuses of Valsalva and aortic annulus outweighs this disadvantage.
Our experience with fusion imaging of CT and conventional angiography is growing. This is commonly used to reduce the risk of vascular complications related to the puncture site and femoral access in all CoreValve implantation procedures, with good results.
ConclusionsTAVI is a promising technique that is undergoing rapid development.
The physiological motion of the cardiac structures during the cardiac cycle means that high-end scanners (minimum 64-slice) are required, generating large volumes of data with sufficient spatial and temporal resolution. MSCT provides precise assessment of the left ventricular outflow tract and complete characterization of the aortic root, aortic valve and aorta, while also enabling accurate identification of the course of the vessels involved in prosthesis delivery, thus anticipating possible complications and obstacles to the procedure.
As the number of TAVI procedures is expected to rise, MSCT will continue to play a central role in the selection of candidates and procedural planning. It may in the future also contribute to the development of prostheses tailored to the individual patient based on prior assessment of the complex structure that is the aortic root.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all staff in the Department of Cardiology whose daily efforts have made it possible to improve our experience in transcatheter aortic valve implantation.