Thirteen years after her last thrombotic event, anticoagulation was discontinued in a patient with combined thrombophilia involving mutation in factor V and G20210A polymorphism of the prothrombin gene. The only history was of arterial thrombosis. Three months later she presented a transmural myocardial infarction caused by coronary thrombosis.
Após treze anos do último evento trombótico, a anticoagulação foi interrompida numapaciente com associação de trombofilia factor V de Leiden e gene mutante da protrombina G20210A. Ela possuía história de tromboses arteriais. Três meses após a paciente apresentouenfarte transmural do miocárdio devido à trombose coronariana.
Genetic, environmental, and behavioral risk factors may interact to cause thrombosis. Factor V Leiden and the prothrombin G20210A mutation are two highly prevalent genetic variants that are well documented risk factors for venous thrombosis. The role of these gene variants in arterial events has been difficult to define. In 1997, two studies by Rosendaal et al.1,2 showed increased frequencies of factor V Leiden and prothrombin G20210A in young women who experienced myocardial infarction (MI). There is a probable synergic role for genetic factors in subgroups of patients presenting with environmental or behavioral factors.
Myocardial infarctions with normal (or near-normal) coronary arteries (MINCA) account for 1% to 12% of cases3,4. A possible mechanism for MINCA is occlusion of the vessel lumen by thrombus that is subsequently rapidly lysed. Etiological factors that have been reported include cocaine use, embolism, coronary endothelial dysfunction and hypercoagulable states.
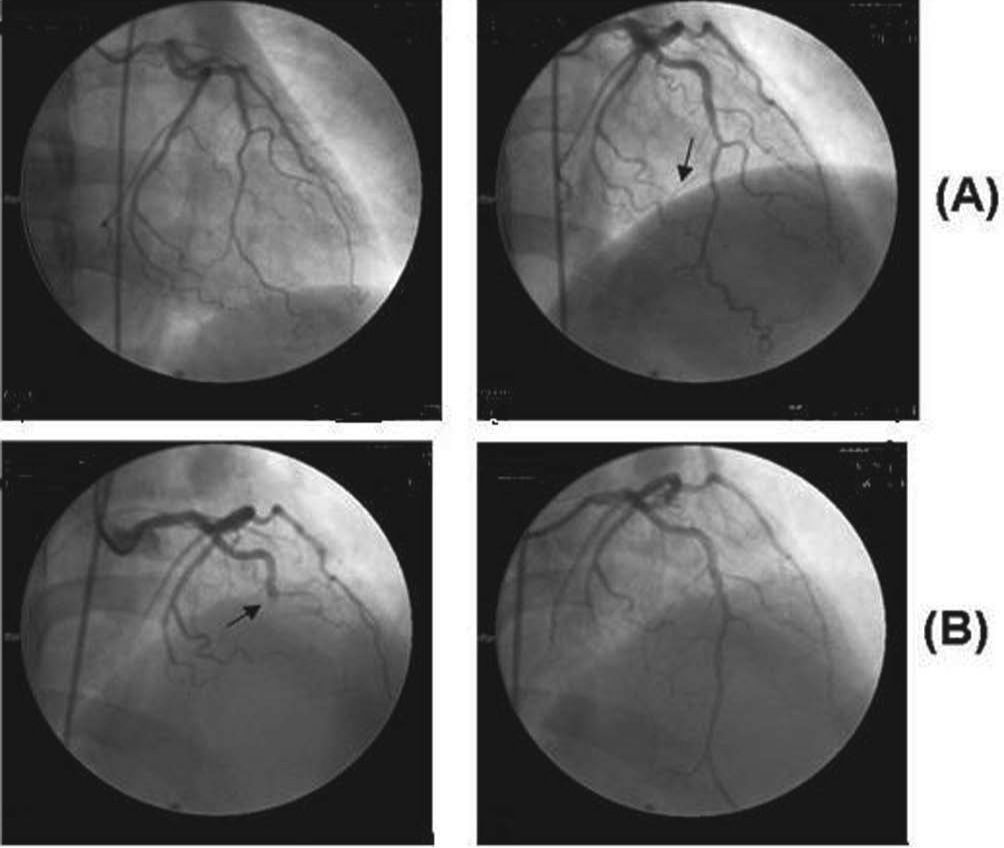
Case reportA 51-year-old white woman was admitted to the hospital with acute chest pain of less than 6 hours duration. The cardiological investigation showed a non-Q wave acute coronary syndrome in the anteroseptal wall that did not affect its motion, and coronary artery angiography showed a normal left anterior descending artery and an occluded distal portion of the third marginal branch of the circumflex artery. She had smoked about 20 to 40 cigarettes/day since the age of 18. No other known risk factors for coronary heart disease were present. The patient was treated with nitroglycerin infusion and an oral beta-blocker. She made a good recovery and was discharged from the hospital two days later. Seventy-two hours later, she experienced severe, squeezing, acute anterior chest pain and was readmitted to the hospital. The ECG showed a pattern typical of a transmural acute anterior wall myocardial infarction. A new coronary angiogram (Figura) showed almost total occlusion of the descending anterior coronary artery and the second diagonal, interpreted as vessels filled with clots, with no vascular lesion (endoluminal irregularities attributed to coronary atherosclerosis). She was treated with analgesics, angioplasty of the two affected vessels without stent placement, abciximab, and a continuous venous infusion of nitroglycerin. After the cessation of abciximab, she was sequentially administered unfractionated heparin, low molecular weight heparin, and warfarin, respectively. At 5-month follow-up, she was being treated with permanent oral anticoagulants in addition to atorvastatin.
Thirteen years before, she had presented with recurrent episodes of transient arterial ischemia of the lower limbs. These arterial events resolved with the use of vasodilator medication. In April 1995, she presented with a mesenteric embolic accident of the superior mesenteric artery which was detected by mesenteric angiography. She received surgery following an embolectomy of the superior mesenteric artery, with apparent recovery of the ischemic segment of the small bowel. Forty-eight hours afterwards, she underwent a second-look operation which revealed a 150cm, non-viable segment of her small bowel, which was resected with transit, and reconstructed with a primary anastomosis. At this time, an investigation for thrombophilic factors was performed, and she was found to be heterozygous for factor V Leiden. Two years later, she was also found to be heterozygous for the prothrombin G20210A variant. Her mother and only sister were heterozygous for factor V Leiden, but negative for prothrombin G20210A. Both her mother and sister smoke, but neither has ever experienced thrombotic events. The patient was maintained on anticoagulant treatment with warfarin for 13 years with no new thrombotic events. She was unable to stop smoking. In March 2007, we decided to discontinue the anticoagulant treatment. Three months afterwards, she presented with the ischemic heart attack.
DiscussionThe association between factor V Leiden and prothrombin G20210A and arterial events is controversial. A study of eight prothrombotic gene polymorphisms, including these two genetic variants, in 1210 patients <45 years of age showed no increased risk of premature MI5. Two meta-analyses6 did not demonstrate an association between factor V Leiden and arterial disease. Nevertheless, in a case–control study1 of young women (aged 18–44), factor V Leiden was associated with a 2.4-fold increased risk of MI. This increased risk was limited to current smokers. There may be a small risk for arterial events associated with these genetic variants, which is considerably amplified when additional risk factors are present.
In a previously reported small series, almost all patients presenting with MINCA were males <50 years of age and regular cigarette smokers3. It has been suggested that thrombophilic factors may increase the risk of MI in patients with normal coronary angiograms more than in those with more severe coronary atherosclerosis1,2. Furthermore, in this specific cardiological situation, the frequencies of both factor V Leiden and prothrombin G20210A seemed to be higher in patients <50 years of age7. In another study8, factor V Leiden was found in 12% of young patients (mean age 44 years) with MINCA, in 4.5% of patients with MI and significant coronary artery disease, and in 5% of normal controls.
It is worth mentioning that carriers of two defects seem to be at greater risk of venous thrombosis than their relatives with a single defect9. A pooled analysis of eight case–control studies10 showed a venous thrombotic risk of 4.9 for factor V Leiden, 3.8 for prothrombin G20210A, and 20 for those heterozygous for both mutations.
The unusual aspect of the present case is that the patient was heterozygous for both genetic variants, but presented with no venous thrombosis, and experienced life-threatening arterial thrombotic events that occurred while she was not receiving anticoagulation.