Hypertension is an important public health problem, affecting about 25% of the adult population worldwide.1 Genetic and environmental factors contribute to its pathogenesis. The T allele of the C825T polymorphism of the beta 3 subunit of G protein (rs5443) leads to the production of a truncated variant that enhances intracellular signaling and may interfere with the regulation of blood pressure. This genetic variant has been described as a risk factor for hypertension, although study results are controversial.
ObjectiveThe objective of this study was to analyze the association of the C825T polymorphism of the GNB3 gene with the occurrence of hypertension in a Portuguese population from the Madeira archipelago.
MethodsA case-control study was performed with 1641 Caucasian individuals (mean age 50.6±8.1 years), 848 with hypertension and 793 controls. Blood was collected from all participants for biochemical and genetic analysis, including genotyping of the C825T polymorphism. Logistic regression analysis was performed to determine which variables were significantly associated with the onset of hypertension. Statistical analyses were performed using IBM SPSS version 19.0 and p-values <0.05 were considered statistically significant.
ResultsIn our study, there was a significant association between the C825T polymorphism of the GNB3 gene and the occurrence of hypertension (odds ratio 1.275; 95% confidence interval 1.042-1.559; p=0.018) in the dominant model, after multivariate analysis.
ConclusionWe conclude that the C825T polymorphism of the beta 3 subunit of G protein is significantly and independently associated with the occurrence of hypertension in the study population.
A hipertensão arterial é um problema de Saúde Pública, afeta 25% da população adulta mundial. Fatores genéticos e ambientais contribuem para a sua patogénese. O polimorfismo C825T da subunidade β3 da Proteína G (rs5443) favorece a produção de uma variante alternativa, truncada, que facilita a sinalização intracelular, pode interferir na regulação da pressão arterial. Essa variante genética tem sido descrita como um fator de risco para a hipertensão arterial, com resultados controversos.
ObjetivoAvaliar a associação do polimorfismo C825T do gene GNβ3 com o aparecimento de hipertensão arterial, numa população portuguesa do Arquipélago da Madeira.
MétodosCom uma amostra de 1.641 indivíduos (média de 50,6 ± 8,1 anos), fizemos um estudo caso-controlo com 848 indivíduos com hipertensão arterial e 793 controlos, ajustados para o sexo e a idade. Todos os participantes colheram sangue para análises bioquímicas e foram genotipados para o polimorfismo C825T. Foi feita uma regressão logística para ver quais as variáveis que se relacionam com a hipertensão arterial. A análise dos dados foi feita com o software estatístico SPSS, versão 19.0. Usamos como limiar de significância o valor de p < 0,05.
ResultadosEncontramos uma associação significativa entre o polimorfismo C825T e o aparecimento de hipertensão arterial (odds ratio = 1,275; IC 95% (1,042–1,559); p = 0,018) no modelo dominante, após análise multivariada.
ConclusãoO polimorfismo C825T da subnidade β3 da Proteína G está associado, de forma significativa e independente, com o aparecimento hipertensão arterial na nossa população.
Hypertension is a major public health problem, affecting around 25% of the adult population worldwide.1 It is a risk factor for cardiovascular disease and progression to renal failure, independently of other cardiovascular risk factors.2,3 In the Portuguese adult population, its overall prevalence is around 42% (44.4% in men and 40.2% in women).4
In recent decades, many studies have been conducted to determine how genetics contributes to the development of hypertension. Around 20-40% of blood pressure variation is genetically determined,5,6 but the molecular genetics of HT is still unclear.
G proteins and their receptors play a crucial role in cell survival. These proteins are part of a superfamily of proteins that, when inactive, are bound to cell membrane receptors, and when activated migrate through the cytosol regulating multiple effectors including enzymes, ion channels, hormones, neurotransmitters and autocrine and paracrine factors.7 They are thus signal transducers that enable intracellular events to be triggered by external stimuli.
Transduction of external stimuli via intracellular signals is clearly of considerable importance for perpetuating viable characteristics on an evolutionary scale. Given their wide-ranging roles, deficiency or altered expression of these important proteins can lead to more or less widespread metabolic disturbances.8
Heterotrimeric G proteins consist of three subunits, alpha, beta and gamma, coded by separate genes. The alpha subunit of each G protein is the most important: it is the subunit that interacts with the receptor, binding to guanosine triphosphate (GTP) and regulating effector systems.9 The beta and gamma subunits are non-covalently bound into the beta-gamma complex, which when bound to the alpha subunit forms the inactive state of the protein.10 In addition to anchoring the alpha subunit, the beta-gamma dimer influences certain cell processes11,12 and can modulate the activity of certain effectors.13 Due to their crucial role in the functioning of many cell types, genetic abnormalities in G protein subunits may be involved in the etiology of a wide range of clinical conditions.
The gene that codes for the G protein beta 3 subunit is located on chromosome 12p13. The C825T single-nucleotide polymorphism of the beta 3 subunit of G protein (rs5443) is located in exon 10 of the GNB3 gene, where cytosine (C) is replaced by thymidine (T) at nucleotide 825. The T allele of the C825T polymorphism results in a splicing variation in exon 9 in which nucleotides 498-620 are deleted,14,15 leading to the absence of amino acids 167 to 197 in the truncated protein (known as GNB3-s). This is a functional protein that enhances protein G-associated intracellular signaling.14,16 Since G-protein activation is the key event in intracellular signal transduction, the C825T polymorphism plays an important role in the etiology of a variety of disease processes,17 including hypertension,14,15,18–20 obesity,21 depression,22 and cardiovascular disease.23 Furthermore, the 825T allele functions as a pharmacogenetic marker to predict responses to various drugs, such as diuretics, antidepressants, sildenafil and clonidine, as well as to angiotensin II and endothelin 1.17
However, the results of some studies have failed to demonstrate any association between this genetic variant and hypertension,24–26 while others support it. In view of this controversy, we decided to assess this association in a Portuguese population.
ObjectiveThe objective of this study was to analyze the association of the C825T polymorphism of the GNB3 gene (rs5443) with the occurrence of hypertension in a Portuguese population from the Madeira archipelago.
MethodsStudy protocol and ethics approvalThe study was approved by the ethics committee of the Health Service of the Autonomous Region of Madeira (SESARAM) and was conducted in accordance with the international ethics guidelines of the 2013 Declaration of Helsinki.
All participants gave their written informed consent, after an internist with more than 10 years of experience explained how blood would be collected for analysis and how DNA and relevant clinical data would be stored and reused. Blood samples for genetic analysis are kept in our hospital's research biobank in accordance with Articles 6, 15 and 16 of the UNESCO Universal Declaration on Bioethics and Human Rights.
Study populationThis study was conducted on the Portuguese archipelago of Madeira, which has a population of around 300000. Participants were Caucasian, born in Madeira, and their parents and grandparents were also Caucasians born in the archipelago. The study sample was thus a genetically homogeneous southern European population.
The sample consisted of 1641 individuals with a mean age of 50.6±8.1 years, 848 (49.9%) male, who were selected sequentially from general and family medicine consultations in the Internal Medicine department of the Central Hospital of Funchal.
The population was divided into two groups, those with and those without hypertension. Differences between the groups in terms of gender and age were kept to a minimum.
HypertensionAt enrollment, subjects were defined as having hypertension if it had already been diagnosed, if they had been taking antihypertensive medication for more than three months, or if they were newly diagnosed with systolic (SBP) and/or diastolic blood pressure (DBP) ≥140/90 mmHg on at least three occasions.27
Individuals with secondary hypertension, such as those with primary hyperaldosteronism or clinically significant renal disease, were excluded, as were those taking medication for other indications that could affect blood pressure.
The normotensive control subjects had never taken antihypertensive medication and had SBP/DBP <140/90 mmHg.
Blood pressure was measured in the right arm with the subject in a seated position after 10 minutes’ rest, using a standard Welch Allyn sphygmomanometer. The mean of three readings taken at 2-min intervals was recorded.27
Data collectionAll participants answered a standardized questionnaire on age, gender, lifestyle, smoking, alcohol consumption, and personal and family history of cardiovascular events, particularly stroke, coronary artery disease and family history of hypertension.
Anthropometric parameters were assessed in all participants. Height was measured in cm and weight in kg, and body mass index (BMI) was calculated as kg/m2.28 BMI was classified as normal (<25 kg/m2), overweight (25-29.9 kg/m2) or obese (≥30 kg/m2).28 Waist circumference was measured and considered high when >94 cm in men and >80 cm in women.29 Waist-hip ratio was defined as high when >0.9 in men and >0.8 in women.29
Pulse wave velocity (PWV) was determined by tonometry using a Complior device, as previously described.30 PWV >10 m/s was considered high. Diabetes was defined as fasting blood glucose ≥126 mg/dl and/or blood glucose ≥200 mg/dl and/or a history of treatment for diabetes.31
A sedentary lifestyle was defined as performing less than 150 min/week of moderate to vigorous physical activity.29
Individuals who smoked at enrollment in the study were considered smokers and were classified according to the number of cigarettes smoked per day: 1-10, 10-20 or more than 20. Individuals who consumed alcohol at enrollment were considered to be drinkers. Alcohol consumption was quantified in g per week and was considered excessive when >70 g/week in men and >50 g/week in women.
Laboratory testsBlood samples were collected after 14-16 hours of fasting and plasma was prepared for the quantification of biochemical parameters. Laboratory tests were performed at the hospital's central laboratory, using standard techniques.
To determine total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides and blood glucose, blood samples were collected in dry tubes, centrifuged for 30 min at 3500 g and quantified by enzymatic techniques using an AU5400 automated analyzer (Beckman Coulter). Biochemical risk markers – lipoprotein(a), apolipoprotein B and high-sensitivity C-reactive protein (hs-CRP) – were quantified with the same analyzer using immunoturbidimetry. Fibrinogen was measured in blood collected into a sodium citrate tube with the subject in a fasting state, using an ACL TOP 700 analyzer (Beckman Coulter).
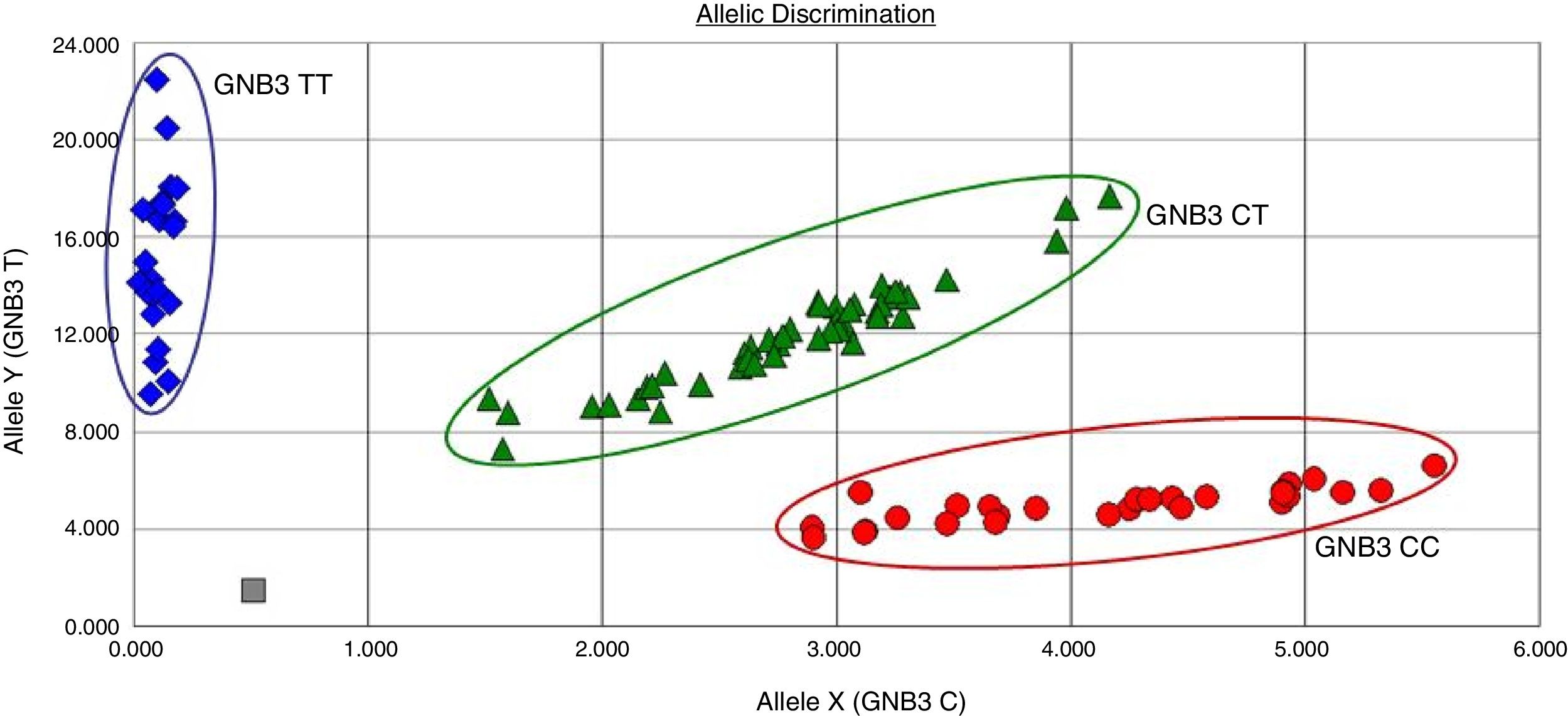
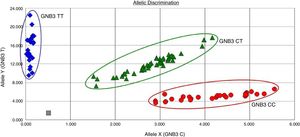
Genetic analysis of the C825T polymorphism of the GNB3 geneGenomic DNA was extracted from peripheral blood leukocytes using the salting-out method. Genotyping was performed using oligonucleotide probes labeled with allele-specific fluorescence in an assay combining standard polymerase chain reaction (PCR) and TaqMan techniques (Applied Biosystems). Primers and probes for the C825T polymorphism of the GNB3 gene (rs5443) were those provided by the manufacturer (TaqMan SNP Genotyping Assays, Applied Biosystems). Oligonucleotides were synthesized and FAM™ and VIC® fluorescent labels were coupled to the 5′ ends of the probes for allelic discrimination. Two-step PCR, consisting of 40 denaturation cycles at 92°C for 15 s and primer annealing and extension at 60°C for 1 min, was carried out in a 7300 Real-Time PCR System (Applied Biosystems). 7300 System SDS Software (Applied Biosystems) was used to determine genotypes, without using prior knowledge of individual clinical data (Figure 1).
Statistical analysisCategorical variables are presented as frequency and percentage using the chi-square test. Continuous variables are expressed as mean ± standard deviation or median (minimum-maximum). The Student's t test or the non-parametric Mann-Whitney test was used to compare continuous variables, as appropriate.
Hardy-Weinberg equilibrium was analyzed for each of the genes using the chi-square test and a p-value less than 5% was considered statistically significant. The Bonferroni correction was applied. Multivariate logistic regression analysis using the forward Wald method was applied to identify variables associated with the onset of hypertension.
The strength of associations was determined by odds ratios (OR) and respective 95% confidence intervals (CIs).
The statistical analysis was performed with IBM SPSS version 19.0 and a p value <0.05 was taken to indicate statistical significance.
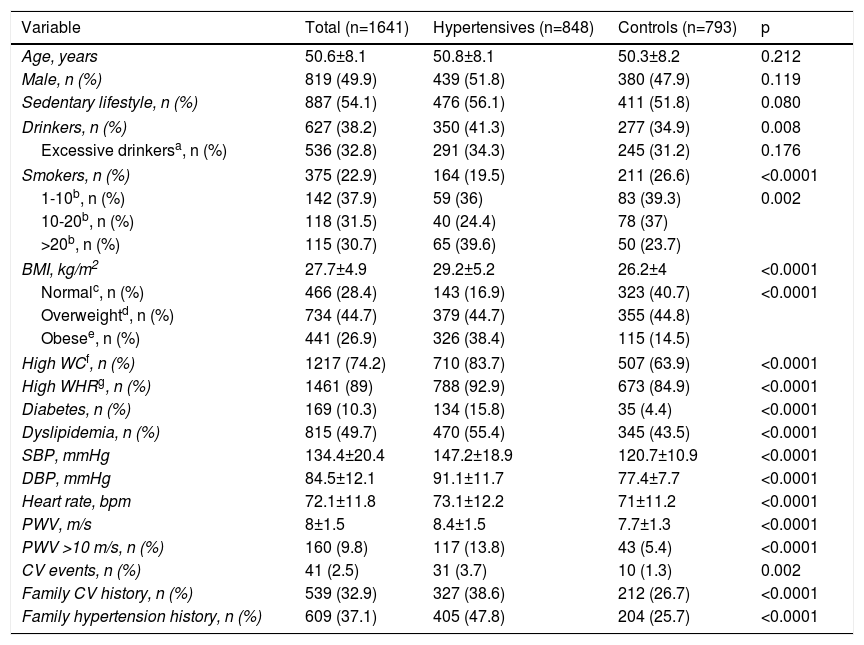
ResultsCharacteristics of the study populationThe baseline characteristics of the study population are presented in Table 1. The sample consisted of 1641 individuals with hypertension (mean age 50.8±8.1 years; 51.8% male) and 793 controls without hypertension (mean age 50.3±8.2 years; 47.9% male). There were no significant differences between the groups in terms of gender or age.
Demographic and clinical characteristics of the study population.
| Variable | Total (n=1641) | Hypertensives (n=848) | Controls (n=793) | p |
|---|---|---|---|---|
| Age, years | 50.6±8.1 | 50.8±8.1 | 50.3±8.2 | 0.212 |
| Male, n (%) | 819 (49.9) | 439 (51.8) | 380 (47.9) | 0.119 |
| Sedentary lifestyle, n (%) | 887 (54.1) | 476 (56.1) | 411 (51.8) | 0.080 |
| Drinkers, n (%) | 627 (38.2) | 350 (41.3) | 277 (34.9) | 0.008 |
| Excessive drinkersa, n (%) | 536 (32.8) | 291 (34.3) | 245 (31.2) | 0.176 |
| Smokers, n (%) | 375 (22.9) | 164 (19.5) | 211 (26.6) | <0.0001 |
| 1-10b, n (%) | 142 (37.9) | 59 (36) | 83 (39.3) | 0.002 |
| 10-20b, n (%) | 118 (31.5) | 40 (24.4) | 78 (37) | |
| >20b, n (%) | 115 (30.7) | 65 (39.6) | 50 (23.7) | |
| BMI, kg/m2 | 27.7±4.9 | 29.2±5.2 | 26.2±4 | <0.0001 |
| Normalc, n (%) | 466 (28.4) | 143 (16.9) | 323 (40.7) | <0.0001 |
| Overweightd, n (%) | 734 (44.7) | 379 (44.7) | 355 (44.8) | |
| Obesee, n (%) | 441 (26.9) | 326 (38.4) | 115 (14.5) | |
| High WCf, n (%) | 1217 (74.2) | 710 (83.7) | 507 (63.9) | <0.0001 |
| High WHRg, n (%) | 1461 (89) | 788 (92.9) | 673 (84.9) | <0.0001 |
| Diabetes, n (%) | 169 (10.3) | 134 (15.8) | 35 (4.4) | <0.0001 |
| Dyslipidemia, n (%) | 815 (49.7) | 470 (55.4) | 345 (43.5) | <0.0001 |
| SBP, mmHg | 134.4±20.4 | 147.2±18.9 | 120.7±10.9 | <0.0001 |
| DBP, mmHg | 84.5±12.1 | 91.1±11.7 | 77.4±7.7 | <0.0001 |
| Heart rate, bpm | 72.1±11.8 | 73.1±12.2 | 71±11.2 | <0.0001 |
| PWV, m/s | 8±1.5 | 8.4±1.5 | 7.7±1.3 | <0.0001 |
| PWV >10 m/s, n (%) | 160 (9.8) | 117 (13.8) | 43 (5.4) | <0.0001 |
| CV events, n (%) | 41 (2.5) | 31 (3.7) | 10 (1.3) | 0.002 |
| Family CV history, n (%) | 539 (32.9) | 327 (38.6) | 212 (26.7) | <0.0001 |
| Family hypertension history, n (%) | 609 (37.1) | 405 (47.8) | 204 (25.7) | <0.0001 |
BMI: body mass index; CV: cardiovascular; DBP: diastolic blood pressure; PWV: pulse wave velocity; SBP: systolic blood pressure; WC: waist circumference; WHR: waist/hip ratio.
Significant differences between groups were seen in alcohol consumption (p=0.008), BMI and obesity (p<0.0001), waist circumference (p<0.0001) and waist/hip ratio (p<0.0001), in all of which mean values were higher in the hypertensive group than in the control group. Likewise, diabetes, dyslipidemia, and high SBP, DBP, heart rate and PWV were significantly more frequent in the hypertensive group than in the normotensive group (p<0.0001). In the hypertensive group, there were more individuals with a personal (p=0.002) and family (p<0.0001) history of cardiovascular events, as well as a family history of hypertension (p<0.0001). There was a significantly larger proportion of smokers in the control group (p<0.0001), except in those who smoked >20 cigarettes/day.
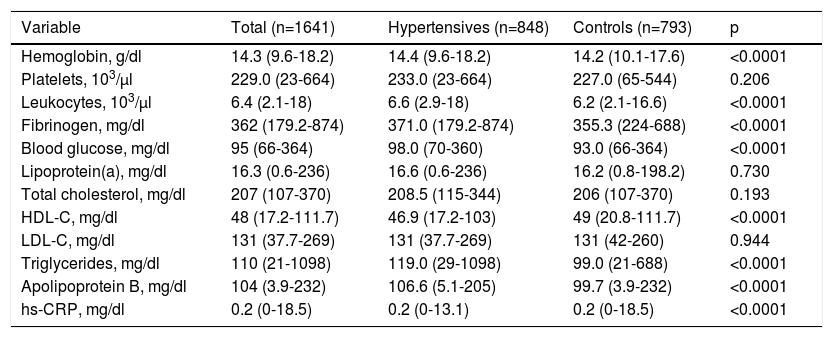
Regarding biochemical variables (Table 2), there were significant differences between the groups in hemoglobin, leukocytes, fibrinogen, HDL cholesterol, triglycerides, apolipoprotein B, fasting blood glucose and hs-CRP (all p<0.0001), with higher mean values in the hypertensive group than in controls. There were no significant differences in relation to total cholesterol, LDL cholesterol, lipoprotein(a) and platelets (p>0.05).
Biochemical characteristics of the study population.
| Variable | Total (n=1641) | Hypertensives (n=848) | Controls (n=793) | p |
|---|---|---|---|---|
| Hemoglobin, g/dl | 14.3 (9.6-18.2) | 14.4 (9.6-18.2) | 14.2 (10.1-17.6) | <0.0001 |
| Platelets, 103/μl | 229.0 (23-664) | 233.0 (23-664) | 227.0 (65-544) | 0.206 |
| Leukocytes, 103/μl | 6.4 (2.1-18) | 6.6 (2.9-18) | 6.2 (2.1-16.6) | <0.0001 |
| Fibrinogen, mg/dl | 362 (179.2-874) | 371.0 (179.2-874) | 355.3 (224-688) | <0.0001 |
| Blood glucose, mg/dl | 95 (66-364) | 98.0 (70-360) | 93.0 (66-364) | <0.0001 |
| Lipoprotein(a), mg/dl | 16.3 (0.6-236) | 16.6 (0.6-236) | 16.2 (0.8-198.2) | 0.730 |
| Total cholesterol, mg/dl | 207 (107-370) | 208.5 (115-344) | 206 (107-370) | 0.193 |
| HDL-C, mg/dl | 48 (17.2-111.7) | 46.9 (17.2-103) | 49 (20.8-111.7) | <0.0001 |
| LDL-C, mg/dl | 131 (37.7-269) | 131 (37.7-269) | 131 (42-260) | 0.944 |
| Triglycerides, mg/dl | 110 (21-1098) | 119.0 (29-1098) | 99.0 (21-688) | <0.0001 |
| Apolipoprotein B, mg/dl | 104 (3.9-232) | 106.6 (5.1-205) | 99.7 (3.9-232) | <0.0001 |
| hs-CRP, mg/dl | 0.2 (0-18.5) | 0.2 (0-13.1) | 0.2 (0-18.5) | <0.0001 |
Values are presented as median (minimum-maximum).
HDL-C: high-density lipoprotein cholesterol; hs-CRP: high-sensitivity C-reactive protein; LDL-C: low-density lipoprotein cholesterol.
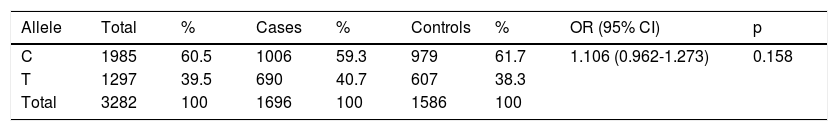
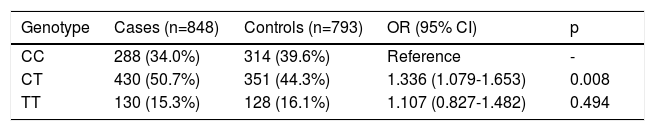
The allele frequencies of the C825T polymorphism of the GNB3 gene are presented in Table 3. The frequencies of the C and T alleles in the overall population were 60.5% and 39.5%, respectively. The proportions were similar in both cases and controls, with OR 1.106 and without statistical significance (p=0.158) (Table 3).
The frequency of each genotype in both groups was in Hardy-Weinberg equilibrium (Table 4). In the additive model, the most frequent genotype was heterozygous CT, with a frequency of 50.7% in the hypertensive group and 44.3% in the controls compared to the reference homozygous CC genotype. This genotype carried a 1.336-fold greater risk of hypertension (p=0.008) than the mutated homozygote TT (OR 1.107; p=0.494).
Genotypes of the C825T polymorphism of the GNB3 gene and risk of hypertension.
| Genotype | Cases (n=848) | Controls (n=793) | OR (95% CI) | p |
|---|---|---|---|---|
| CC | 288 (34.0%) | 314 (39.6%) | Reference | - |
| CT | 430 (50.7%) | 351 (44.3%) | 1.336 (1.079-1.653) | 0.008 |
| TT | 130 (15.3%) | 128 (16.1%) | 1.107 (0.827-1.482) | 0.494 |
CI: confidence interval; OR: odds ratio.
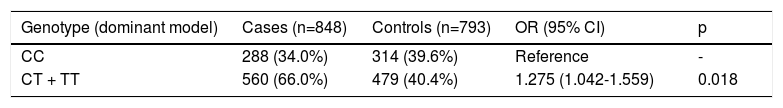
In the dominant model (Table 5), subjects with the CT or TT genotype were at greater risk of developing hypertension (OR 1.275; 95% CI 1.042-1.559) than those with the reference genotype (CC) (p=0.018).
Genotypes of the C825T polymorphism of the GNB3 gene and risk of hypertension (dominant model).
| Genotype (dominant model) | Cases (n=848) | Controls (n=793) | OR (95% CI) | p |
|---|---|---|---|---|
| CC | 288 (34.0%) | 314 (39.6%) | Reference | - |
| CT + TT | 560 (66.0%) | 479 (40.4%) | 1.275 (1.042-1.559) | 0.018 |
CI: confidence interval; OR: odds ratio.
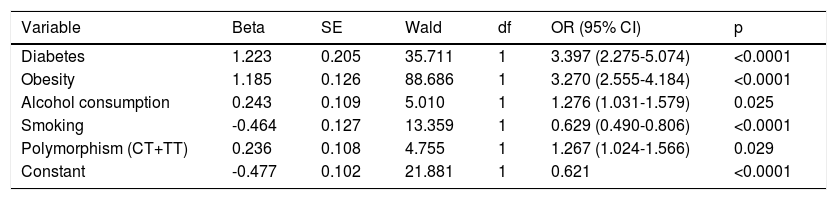
In the logistic regression analysis (Table 6), the variables that remained in the model were diabetes, obesity, alcohol consumption and the CT and TT genotypes of the C825T polymorphism of the GNB3 gene, which were significantly and independently associated with the occurrence of hypertension.
Logistic regression analysis of the C825T polymorphism of the GNB3 gene and confounding variables.
| Variable | Beta | SE | Wald | df | OR (95% CI) | p |
|---|---|---|---|---|---|---|
| Diabetes | 1.223 | 0.205 | 35.711 | 1 | 3.397 (2.275-5.074) | <0.0001 |
| Obesity | 1.185 | 0.126 | 88.686 | 1 | 3.270 (2.555-4.184) | <0.0001 |
| Alcohol consumption | 0.243 | 0.109 | 5.010 | 1 | 1.276 (1.031-1.579) | 0.025 |
| Smoking | -0.464 | 0.127 | 13.359 | 1 | 0.629 (0.490-0.806) | <0.0001 |
| Polymorphism (CT+TT) | 0.236 | 0.108 | 4.755 | 1 | 1.267 (1.024-1.566) | 0.029 |
| Constant | -0.477 | 0.102 | 21.881 | 1 | 0.621 | <0.0001 |
Forward Wald method in SPSS version 19.0. Sedentary lifestyle, gender and age left the equation. Statistical significance for p<0.05.
CI: confidence interval; df: degrees of freedom; OR: odds ratio; SE: standard error.
The model showed smoking to be a protective factor, while a sedentary lifestyle left the model and did not influence the occurrence of hypertension.
DiscussionIn the present study, we investigated the association between the 825T variant of the GNB3 G protein gene and hypertension in a Portuguese population from the Madeira archipelago. Subjects with the dominant genetic model had an increased risk of developing hypertension (OR 1.275; 95% CI 1.042-1.559; p=0.018), even after multivariate analysis and correction for confounding factors (obesity, diabetes, sedentary lifestyle, alcohol consumption and smoking). This demonstrates that in this population G protein variants are independently associated with hypertension, constituting an additional risk to conventional risk factors.
The frequency of the T allele was 39.5% in our study, which is higher than that reported in other Caucasian populations (37.7%), but lower than that described for Asians (48.4%) and African-Americans (76.3%).19
Our results, which are innovative in the Portuguese population, are in line with those of other studies. Stiffer et al.,14 in a study of 427 normotensive and 426 hypertensive German subjects, were the first to demonstrate a significant association between the C825T polymorphism of the GNB3 gene coding for pertussis toxin-sensitive G proteins and hypertension (OR 1.79; 95% CI 1.05-3.05; p=0.03). Subsequently, other studies in different countries and ethnic groups also found associations between the C825T polymorphism and hypertension14,15,17–19 and variations in blood pressure.32–34
In a study of Caucasian subjects, Benjafield et al.15 concluded that the 825T allele was associated with hypertension (OR 2.3; 95% CI 1.5-3.7),15 while a study in an Egyptian population also found an association between this variant and hypertension (OR 1.4; 95% CI 0.9-2.1) in the dominant genetic model, which was significantly stronger in the recessive model (OR 2.3; 95% CI 1.5-3.7).20
Zheng et al.19, in a meta-analysis involving 36802 individuals, confirmed a significant association between the C825T polymorphism and overall risk of hypertension in Caucasian and Chinese subjects, although this association was not found in other Asian populations.19
By contrast, a meta-analysis by Guo et al. suggested that although the 825T allele showed a marginal association with hypertension risk, there was no association with hypertension in Asians or Caucasians.26
Various pathophysiological mechanisms may explain the role of the C825T (rs5443) genetic variant in the development of hypertension.
Siffert et al.14 reported that the splice variant GNB3-s is a functional protein that increases G protein activity in a reconstituted system and leads to enhanced intracellular signaling.
Elevated blood pressure is due to increased sensitivity to vasopressor hormones, which use GNB3 for signal transmission.35 This has been demonstrated in vivo by the greater vascular reactivity shown by individuals with the 825T variant following stimulation of coronary alpha2 adrenoreceptors.36
Meirhaeghe et al.16 assessed coronary artery vasomotility after intravenous injection of methylergonovine maleate, a vasoconstrictor, and concluded that subjects bearing at least one T allele of the C825T polymorphism had greater susceptibility to vasoconstriction than those with the wild-type CC genotype. The GB3-s subunit is associated with increased G protein activity and therefore may increase the activity of alpha-1A adrenoceptors coupled to these proteins. This would explain the greater vasoconstriction observed in response to a vasoconstrictor in individuals with the 825T allele.
Another hypothesis, put forward by Siffert et al.,14 is that the onset of hypertension in carriers of the T allele may result not from increased vasoconstriction due to vasoconstrictor hormones such as noradrenaline and angiotensin II activating heterotrimeric non-pertussis toxin-sensitive G proteins, but from vascular smooth muscle cell proliferation leading gradually to vascular hypertrophy.
We performed logistic regression analysis to assess confounding variables such as diabetes, alcohol consumption, smoking and the dominant model of the C825T polymorphism, in order to determine which variables were significantly and independently related to the occurrence of hypertension. Obesity and diabetes conferred a significant risk (OR 3.270; 95% CI 2.555-4.184; p<0.0001 and OR 3.397; 95% CI 2.275-5.074; p<0.0001, respectively). The C825T polymorphism also remained in the model, which revealed that it was significantly and independently associated with the occurrence of hypertension (OR 1.276; 95% CI 1.024-1.566; p=0.029).
In our study population there was a larger proportion of smokers (except those who smoked >20 cigarettes/day) in the control group than in the hypertensive group. Multivariate analysis revealed that smoking appeared to be a protective factor for the occurrence of hypertension. This is because smokers were defined as those who smoked at enrollment in the study, so these individuals were active rather than former smokers. Hypertensive individuals were already under medical monitoring for cardiovascular risk factors at enrollment, which would have led some of them to quit smoking. However, our results indicate that the heaviest smokers found it most difficult to quit.
Obesity is also known to be an important risk factor for the development of hypertension. Cross-sectional studies have shown that obesity is associated with higher blood pressure and weight gain throughout life and is an important predictor of hypertension.37–40 Studies in different ethnic groups have shown that the T allele of the C825T polymorphism is associated with obesity41,42 and can influence blood pressure variations.43,44
Future studies that correlate abnormalities in complex cellular transduction mechanisms with other pathological processes may lead to important discoveries in the pharmacological treatment of hypertension and many other diseases.
Strong points of the studyThis is the first case-control study conducted in the genetically homogeneous45–47 and relatively isolated Portuguese population of the archipelago of Madeira to investigate the association between the C825T polymorphism of the GNB3 gene and propensity for hypertension.
The population's characteristics are advantageous for gene mapping in rare disorders, and furthermore, according to several researchers, the study of a culturally and genetically isolated population with a common way of life, diet and natural environment can help minimize the influence of environment factors on variation in disease patterns.48
ConclusionIn this study, the C825T polymorphism of the GNB3 gene was significantly and independently associated with the occurrence of hypertension in a Portuguese population (OR 1.275; 95% CI 1.042-1.559; p=0.018) in the dominant model, even after logistic regression analysis which included other variables that could also have been associated with the onset of hypertension.
Identifying candidate genes and understanding their function will help establish a specific molecular basis for hypertension. Further studies in different populations and with larger sample sizes are needed to determine whether the C825T polymorphism plays a significant role in the etiology of hypertension.
FundingThis study was funded by the Operational Program for the Enhancement of Economic Potential and Territorial Cohesion of the Autonomous Region of Madeira (INTERVIR+).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sousa AC, Palma dos Reis R, Pereira A, Borges S, Gouveia S, Spínola A, et al. A variante genética c825t da subunidade β3 da proteína G associa-se com a hipertensão arterial numa população portuguesa. Rev Port Cardiol. 2018;37:499–507.