Implantable cardioverter-defibrillators (ICDs) are important tools in the prevention of sudden death, but implantation requires transvenous access, which is associated with complications. Subcutaneous implantable cardioverter-defibrillators (S-ICDs) may prevent some of these complications.
AimTo evaluate the therapeutics and complications associated with S-ICD systems.
MethodsS-ICD implantation was planned in 23 patients, for whom the indications were vascular access problems, increased risk of infection or young patients with long predicted follow-up. The population consisted of four patients with ischemic heart disease, three of them on hemodialysis (two with subclavian vein thrombosis), five with left ventricular noncompaction, four with Brugada syndrome, three with arrhythmogenic right ventricular cardiomyopathy, one with transposition of the great vessels, two with dilated cardiomyopathy and four with hypertrophic cardiomyopathy.
ResultsS-ICDs were implanted in 21 patients, two having failed to fulfill the initial screening criteria. Mean implantation time was 77 minutes, with no complications. Defibrillation tests were performed, and in one patient the generator had to be repositioned to obtain an acceptable threshold.
In a mean follow-up of 14 months, 10 patients had S-ICD shocks, which were appropriate in half of them; one developed infection, one needed early replacement due to loss of telemetry and one patient died of noncardiac cause.
ConclusionsS-ICD implantation can be performed by cardiologists with a high success rate. Initial experience appears favorable, but further studies are needed with longer follow-up times to assess the safety and efficacy of this strategy compared to conventional devices.
Os cardioversores-desfibrilhadores implantáveis (CDI) são ferramentas essenciais na prevenção de morte súbita. Requerem a utilização de acessos transversos com as inerentes complicações. O cardioversor-desfibrilhador totalmente subcutâneo (CDI-SC) pode prevenir algumas destas complicações.
ObjetivoAvaliação das terapêuticas e complicações associadas ao sistema.
População e métodosEm 23 doentes houve intenção de implantar CDI-SC. As indicações foram: problemas com acesso vascular, risco de infeção aumentado ou jovens com previsão de prolongado tempo de seguimento. O dispositivo foi implantado em quatro doentes com cardiopatia isquémica, três deles em hemodiálise (dois destes doentes com trombose da veia subclávia). Em cinco jovens com ventrículo esquerdo não compactado, quatro com síndrome de Brugada, três com miocardiopatia arritmogénica do ventrículo direito, um com transposição dos grandes vasos, dois com miocardiopatia dilatada e quatro com miocardiopatia hipertrófica.
ResultadosFoi possível a implantação em 21 doentes. Dois doentes não apresentaram screening inicial do ECG de superfície compatível com vetores de desfibrilhação. O tempo médio de implantação foi 77 minutos, sem complicações. Foi efetuado teste de desfibrilhação; num dos doentes foi necessário reposicionar o gerador para obter um limiar aceitável.
Num seguimento médio de 14 meses, dez doentes apresentaram choques, sendo que em metade foram apropriados, um apresentou infeção do sistema, um teve necessidade de substituição precoce por perda de telemetria e um morreu por causa não cardíaca.
ConclusõesA implantação de CDI-SC é passível de ser efetuada por cardiologistas, com taxa de sucesso elevada. A experiência inicial parece favorável, mas são necessários estudos adicionais com tempos de seguimento mais longos para avaliar a segurança e eficácia desta estratégia em comparação com a convencional.
Implantable cardioverter-defibrillators (ICDs) are effective in primary and secondary prevention of sudden death from ventricular arrhythmias.1–4 Since the first ICD was implanted in the 1980s, technological advances and greater availability have led to a widening of clinical indications, which are specified in European Society of Cardiology guidelines on prevention of sudden death, cardiac devices and heart failure.1,3
These devices require transvenous access to implant one or more leads in the cardiac chambers.5 Their weakest link is the transvenous pacing leads, which are associated with problems of implantation (thrombosis at the access site), longevity, infection and fracture, as well as acute procedure-related complications such as pneumothorax, hemothorax and cardiac tamponade.6 Such complications are often responsible for inappropriate therapies or for preventing appropriate therapies, necessitating further intervention to replace the system. Despite technological advances in generators and leads in recent decades, the use of transvenous access for ICD implantation has a high morbidity rate.6
A defibrillator system has recently been developed that is totally extravascular, designed to reduce the morbidity associated with transvenous pacing leads. The present study describes our center's experience of this device in 21 patients with a mean follow-up of over one year.
MethodsOf a total of 1167 patients undergoing ICD implantation for primary or secondary prevention in our center between December 2008 and January 2013, 21 received S-ICDs.
Patient selection was based on the possible advantages of such a system for young individuals with a high probability of requiring system replacement and for patients with vascular access problems and/or high risk of infection.
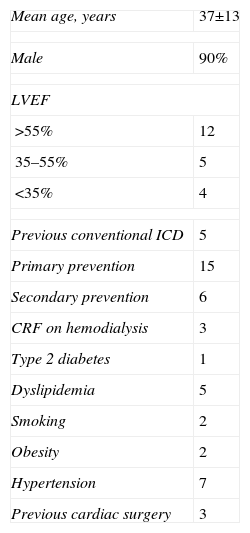
S-ICD implantation was planned in 23 patients, but two failed to fulfill the initial screening criteria, one with Brugada syndrome and the other with left ventricular noncompaction (LVNC), and so 21 patients received S-ICDs. The mean age of the population was 37±13 years and 19 were male. Cardiovascular risk factors included hypertension, type 2 diabetes, obesity, smoking and dyslipidemia, as shown in Table 1.
Demographic and clinical characteristics of the population.
| Mean age, years | 37±13 |
| Male | 90% |
| LVEF | |
| >55% | 12 |
| 35–55% | 5 |
| <35% | 4 |
| Previous conventional ICD | 5 |
| Primary prevention | 15 |
| Secondary prevention | 6 |
| CRF on hemodialysis | 3 |
| Type 2 diabetes | 1 |
| Dyslipidemia | 5 |
| Smoking | 2 |
| Obesity | 2 |
| Hypertension | 7 |
| Previous cardiac surgery | 3 |
CRF: chronic renal failure; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction.
Most patients had preserved ejection fraction. Six underwent S-ICD implantation for secondary prevention, five had conventional ICDs that required replacement (due to endocarditis of the system in two cases and lead fracture in the other three), and three had undergone previous cardiac surgery (coronary artery bypass grafting, correction of transposition of the great vessels, and Morrow procedure in a patient with hypertrophic cardiomyopathy).
The devices were implanted in four patients with ischemic heart disease, three of them with chronic renal failure on hemodialysis (two with subclavian vein thrombosis) and hence increased risk of infection, and the fourth with infection of a previously implanted conventional system; four young patients with a clinical diagnosis of LVNC and a history of nonsustained ventricular tachycardia (VT) on Holter monitoring or exercise testing, syncope or a family history of sudden death (all with an echocardiographic diagnosis confirmed by magnetic resonance imaging); three young adults with Brugada syndrome; three with arrhythmogenic right ventricular cardiomyopathy (ARVC); one with transposition of the great vessels; two with dilated cardiomyopathy; and four with hypertrophic cardiomyopathy, one of whom had undergone previous cardiac surgery and had a family history of sudden death (Table 2).
Patients (n=21) undergoing S-ICD implantation.
| Age | Underlying disease | Implantation time (min) | Previous ICD | Primary prevention | Appropriate shocks | Inappropriate shocks | VT | VF | Time to shock (s) |
| 37 | Brugada | 60 | N | Y | N | N | |||
| 34 | Brugada | 60 | N | Y | N | N | |||
| 46 | Brugada | 100 | Y | Y | N | N | |||
| 31 | ARVC | 69 | Y | N | Y | N | Y | 13 | |
| 18 | ARVC | 59 | N | N | Y | N | Y | 12 | |
| 21 | ARVC | 60 | N | Y | N | Y | |||
| 61 | IHD | 70 | N | N | Y | N | Y | Y | 16 |
| 48 | IHD | 75 | N | N | Y | N | Y | 13 | |
| 65 | IHD | 61 | Y | Y | N | N | |||
| 56 | IHD | 47 | N | Y | N | N | |||
| 72 | DCM | 68 | N | N | N | N | |||
| 42 | DCM | 61 | N | Y | N | N | |||
| 21 | HCM | 120 (1st implantation) | Y | Y | N | Y | |||
| 23 | HCM | 57 | N | Y | N | Y | |||
| 23 | HCM | 63 | N | Y | N | N | |||
| 36 | HCM | 55 | N | Y | N | N | |||
| 17 | LVNC | 80 | N | Y | Y | N | Y | 14 | |
| 27 | LVNC | 90 | N | Y | N | N | |||
| 17 | LVNC | 53 | N | Y | N | Y | |||
| 25 | LVNC | 80 | N | Y | N | N | |||
| 57 | TGV | 100 (with epicardial pacemaker) | Y | N | N | Y |
ARVC: arrhythmogenic right ventricular cardiomyopathy; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; ICD: implantable cardioverter-defibrillator; IHD: ischemic heart disease; LVNC: left ventricular noncompaction; N: no; TGV: transposition of the great vessels; VF: ventricular fibrillation; VT: ventricular tachycardia; Y: yes.
As in conventional ICD implantation, all patients were admitted on the day of the procedure and discharged on the following day. Previously prescribed medication was not changed, including anticoagulants if therapeutic levels were confirmed (one patient was under warfarin therapy). Mean procedure time was 77±14 min.
All devices were implanted in the electrophysiology and pacing unit, with the exception of one case that required an epicardial pacemaker, which was performed in the surgical block with the support of the cardiothoracic surgical team.
The S-ICD system used (SQ-RX 1010, Cameron Health®) consists of a pulse generator and a tripolar lead that records the electrical activity of the heart. It has three vector projections of electrical conduction, with three sensing vector positions: primary, secondary and alternate. The tachycardia detection algorithm is based on heart rate and QRS complex morphology. If heart rate rises above the minimum programmed for detection, further analysis based on QRS morphology assesses the need for therapy, which consists of up to five 80-J shocks, with potential transthoracic back-up pacing for 30 s.
All patients undergo initial screening, in which a surface ECG with leads placed in the areas corresponding to the subcutaneous sensing vectors is analyzed using a specially designed visual guide to assess the amplitude and duration of the QRS and T wave, in order to exclude double counting.
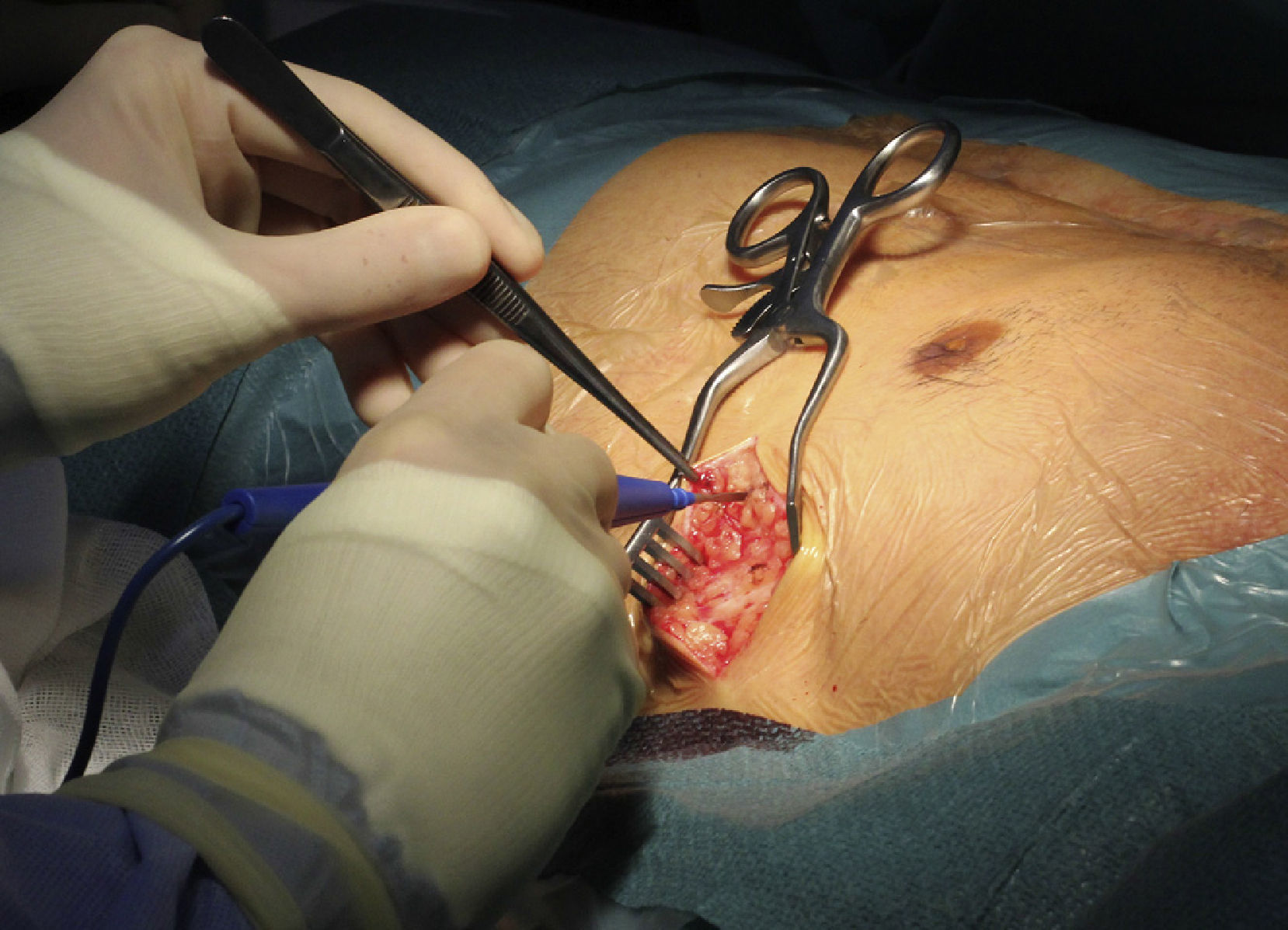
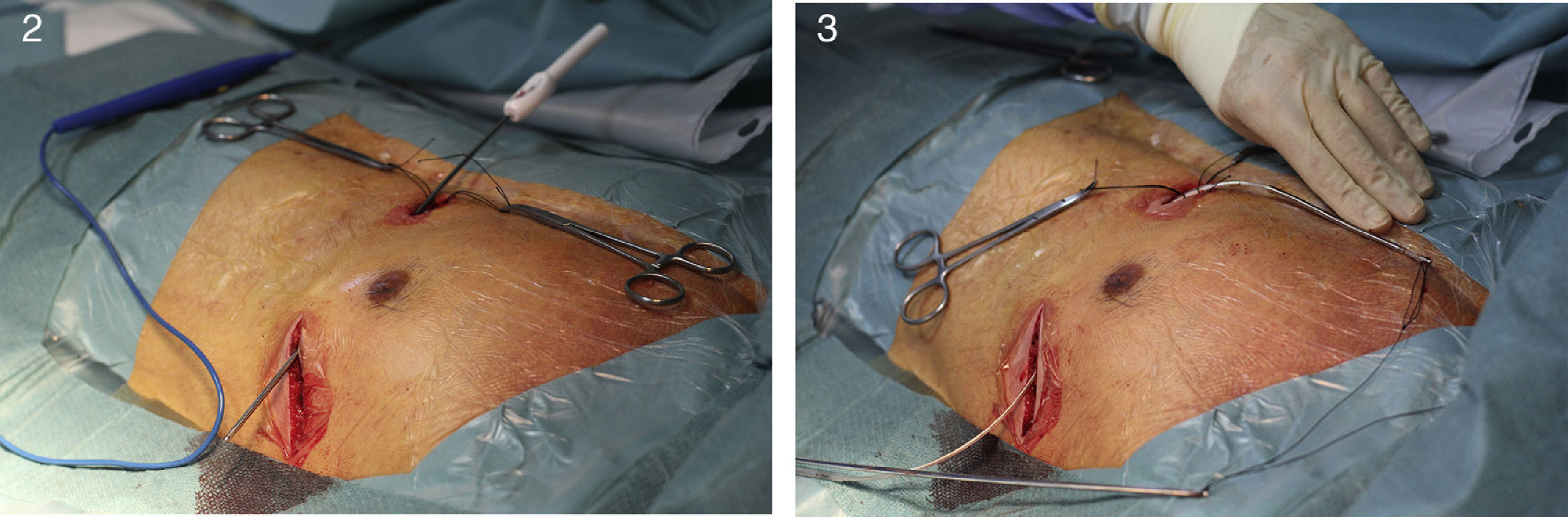
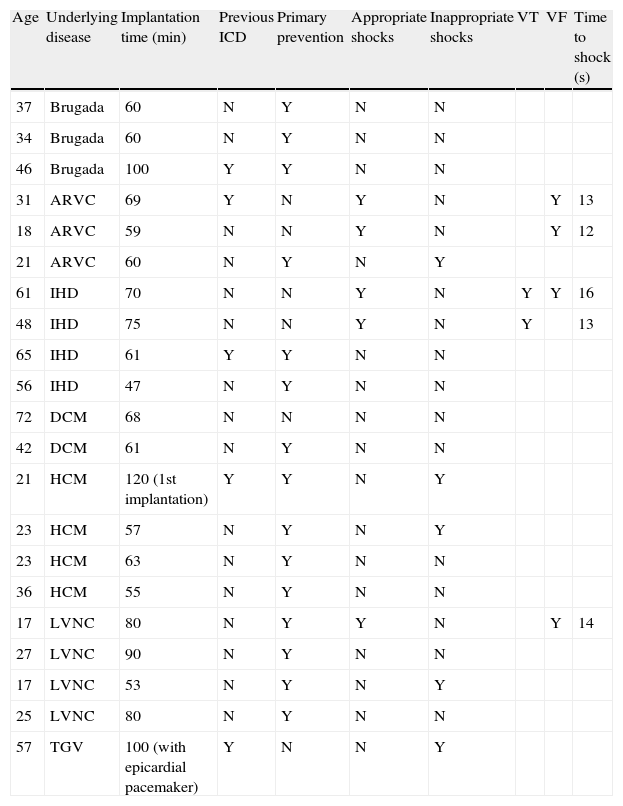
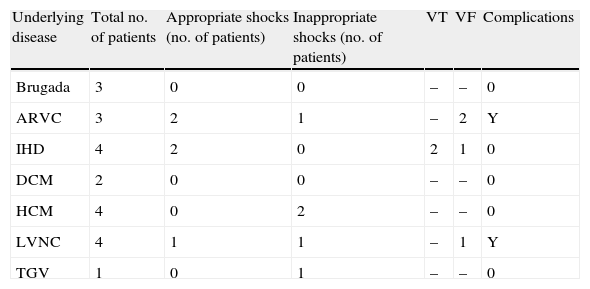
Implantation does not require fluoroscopy, since it is guided by anatomical landmarks. The protocol includes sedation and anesthesia (propofol 2 mg/kg/h and remifentanil 0.2 μg/kg/min) and prophylactic antibiotic therapy (ceftriaxone). The generator pocket is created at the left mid-axillary line in the vicinity of the sixth intercostal space (Figure 1). The defibrillator lead is implanted adjacent to the left sternal border, and following an initial incision in the xiphoid appendix, the lead is tunneled to the pocket, and then to the sternal manubrium (Figures 2 and 3).
Defibrillation tests at 65 J were performed in all patients immediately after implantation. There were no procedure-related complications.
Patients were observed and a chest X-ray performed before hospital discharge, and they were assessed at 1, 3, 6 and 12 months after discharge, and whenever they received ICD therapies.
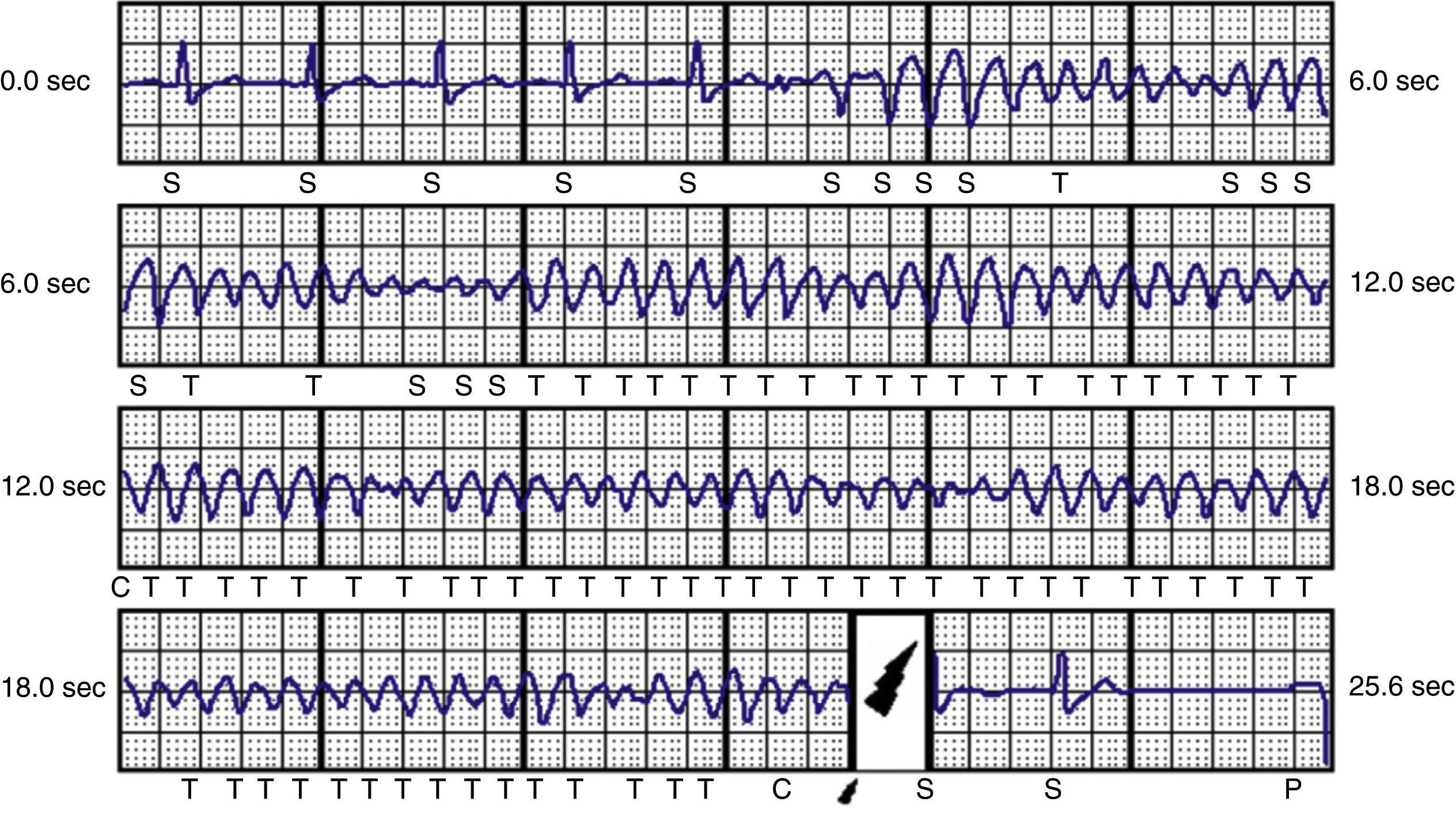
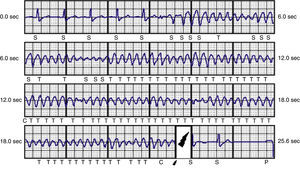
ResultsDuring a mean follow-up of 14 months, one patient required repositioning of the generator following a failed defibrillation test. In one of the patients with ARVC, there was a loss of telemetry after an appropriate therapy on the day of device implantation; the device was replaced by a conventional system on the following day and S-ICD procedures were suspended in our center until clarification was received from the manufacturer. After analyzing the situation, the manufacturer issued a product advisory reporting three cases in Europe of battery-related problems, which were solved by a software upgrade and no further cases have been reported. The oldest patient (72 years), who received an S-ICD due to idiopathic dilated cardiomyopathy and subclavian vein thrombosis, died of non-cardiovascular cause (sepsis due to Clostridium difficile), no episodes being recorded up to the time of death. Five patients received inappropriate shocks, two due to sinus tachycardia during sports activities and three due to T-wave oversensing, necessitating reprogramming of the secondary vector; no further therapies were recorded after the change. Two patients with ischemic heart disease and chronic renal failure on hemodialysis presented episodes of VT and ventricular fibrillation (VF) (Figure 4), which were successfully treated by appropriate shocks; the patient with VF subsequently underwent VF ablation and suffered no further episodes. Two patients with ARVC and a third with LVNC also had appropriate therapies for VF (Tables 2 and 3).
Number of shocks for arrhythmias in the study population.
| Underlying disease | Total no. of patients | Appropriate shocks (no. of patients) | Inappropriate shocks (no. of patients) | VT | VF | Complications |
| Brugada | 3 | 0 | 0 | – | – | 0 |
| ARVC | 3 | 2 | 1 | – | 2 | Y |
| IHD | 4 | 2 | 0 | 2 | 1 | 0 |
| DCM | 2 | 0 | 0 | – | – | 0 |
| HCM | 4 | 0 | 2 | – | – | 0 |
| LVNC | 4 | 1 | 1 | – | 1 | Y |
| TGV | 1 | 0 | 1 | – | – | 0 |
ARVC: arrhythmogenic right ventricular cardiomyopathy; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; LVNC: left ventricular noncompaction; TGV: transposition of the great vessels; VF: ventricular fibrillation; VT: ventricular tachycardia; Y: yes.
There were no acute procedure-related complications, including lead dislocation or fracture.
DiscussionAs demonstrated in various trials and meta-analyses, ICD implantation is indicated in patients at high risk of sudden death.7–9 Nevertheless, there are a growing number of reports of problems surrounding the implantation and use of these devices, both mechanical and programming-related,10,11 including endocarditis, bleeding, venous thrombosis and pneumothorax.6,10 Transvenous lead fracture or dislocation and increased thresholds are common complications that become more frequent over time, even the series with the best results showing only 80% of leads functioning 10 years after implantation.10 In order to minimize these risks, it is essential to consider systems that require less invasive implantation and are easier to replace.
The risk of vascular complications is lower with S-ICDs,12 and so they can be used in patients with congenital heart disease, extensive thrombotic lesions or a predisposition for infection. They are thus of particular interest for those with previously implanted conventional ICDs who have suffered vascular complications or infections. S-ICDs do not limit arm movements and patients recover rapidly after implantation, which makes them especially suitable for younger and more active individuals. They also have excellent arrhythmia discrimination performance, as demonstrated by the START study, which showed 100% sensitivity for VF detection.13
The main limitation of S-ICD systems is their inability to provide antibradycardia and antitachycardia pacing. During patient selection, we therefore excluded those with symptomatic sinus bradycardia, any degree of atrioventricular block or monomorphic VT likely to respond to antitachycardia pacing. In our series, only two appropriate shocks (in different patients) were triggered by VT, all the others being due to VF, and both these patients had ischemic heart disease. This limitation is less important when the target population for S-ICD implantation consists of children or young adults in whom the main arrhythmia is VF or high-rate VT that is unlikely to respond effectively to antitachycardia pacing.
The voltage gradient of the shocks delivered by S-ICDs is lower than that of conventional systems (4 V/cm vs. 30 V/cm) and distributed more evenly, reducing the elevation of troponin levels and depression of contractility associated with therapies.14 Once the advantages of using a single detection zone to deliver high-rate therapy have been confirmed,15 the indications for S-ICD implantation in patients requiring a defibrillator for primary prevention may be extended. In addition, the lack of pacing can be overcome by implanting an epicardial or even transvenous pacemaker.16 In our study, the patient with transposition of the great vessels received an S-ICD following removal of a conventional ICD due to endocarditis, together with an epicardial pacemaker, with no complications.
ConclusionIn our experience, S-ICDs appear to be an efficacious and safe alternative to transvenous systems.
More widespread use of these devices in the future will help determine whether they are suitable for all patients with indication for an ICD or whether they should be reserved for selected patients.
S-ICD implantation can be performed by cardiologists, with a high success rate and similar procedure times to those for conventional ICDs.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Galvão P, Cavaco D, Adragão P, et al. Cardioversor desfibrilhador implantável subcutâneo – experiência de um centro. Rev Port Cardiol. 2014;33:511–517.