The aim of this study was to detect abnormalities in left ventricular myocardial function due to HIV (human immunodeficiency virus) infection without established cardiovascular disease.
MethodsAn echocardiogram was performed in 50 asymptomatic HIV-infected patients (age 41±6 years, 64% male) and in 20 healthy individuals. Conventional echocardiography and pulsed tissue Doppler imaging (TDI) were performed according to the guidelines. The strain rate of the basal segments was obtained with color tissue Doppler and used to evaluate systolic strain rate (SRS), early diastolic strain rate (SRE) and late diastolic strain rate (SRA). Longitudinal, radial and circumferential strain were assessed by 2D speckle tracking.
ResultsThe mean duration of HIV infection was 10±5 years, CD4 count was 579±286 cells/mm3, 32% had detectable viral load, and 86% were under treatment. Of the HIV-infected patients, one had grade 1 diastolic dysfunction. The groups were not different except for E wave (HIV 0.72±0.17 m/s vs. control 0.84±0.16 m/s, p=0.01), longitudinal strain (−19.5±1.9% vs. −21±2%, p=0.005), SRS (−1.1±0.28 s−1 vs. −1.3±0.28 s−1, p=0.02) and SRE (1.8±0.4 s−1 vs. 2.2±0.4 s−1, p<0.001), but only SRS (p=0.03, 95% CI 0.036; 0.67) and SRE (p=0.001, 95% CI −0.599; −0.168) had independent value.
ConclusionIn an HIV-infected population without established cardiovascular disease, myocardial deformation abnormalities can be detected with strain and strain rate, revealing markers of myocardial injury.
Determinar alterações na função miocárdica secundárias à infeção do vírus da imunodeficiência humana (VIH) em doentes sem doença cardiovascular estabelecida.
MétodosForam realizados ecocardiogramas em 50 doentes com infeção VIH, assintomáticos (41±6 anos, 64% homens) e em 20 indivíduos saudáveis. O ecocardiograma convencional e o Doppler tecidular pulsado foram realizados e determinados segundo as últimas recomendações. O strain rate dos segmentos basais foram determinados com o Doppler tecidular cor e avaliados o strain rate sistólico (SRS), o strain rate diastólico precoce (SRE) e o strain rate diastólico tardio (SRA). O strain longitudinal, radial e circunferencial foi obtido a partir do 2D speckle tracking.
ResultadosNa amostra a duração da infeção era de 10±5 anos, com contagem de CD4 de 579±286 células/mm3, 32% tinham carga viral detetável e 86% estavam em terapêutica ativa. No grupo infetado, um doente tinha disfunção diastólica de grau 1. Os grupos eram semelhantes, exceto para a onda E (VIH 0,72±0,17 m/s versus control 0,84±0,16 m/s, p=0,01), strain longitudinal (–19,5±1,9% versus –21±2%, p=0,005), SRS (–1,1±0,28 s-1 versus –1,3±0,28 s-1, p=0,02) e SRE (1,8±0,4 s-1 versus 2,2±0,4 s-1, p<0,001), mas só o SRS (p=0,03, 95% CI 0,036; 0,67) e o SRE (p=0,001, 95%CI –0,599; –0,168) foram diferentes na análise multivariada.
ConclusãoNuma população infetada com o VIH, mas sem doença cardiovascular estabelecida, as alterações na deformação miocárdica podem ser apercebidas através do strain e do strain rate, revelando possíveis marcadores de lesão miocárdica.
In Portugal the incidence of HIV infection is increasing, with more than 6000 new cases per year, and mortality is decreasing (less than 500 deaths).1 With greater longevity, mostly due to newer and more effective antiretroviral therapies and antimicrobial prophylaxis, mortality and morbidity have shifted from opportunistic illness toward a variety of other medical conditions,2–5 including cardiovascular disease,6–10 which occur later in the course of HIV infection.11–13
Cardiovascular disease in HIV infection is diverse. Myocarditis, pericarditis, pleural effusion or neoplastic invasion may be due to opportunistic infections and tumors,14 directly dependent on immunological status. On the other hand, coronary artery disease has a complex and multifactorial pathogenesis, and the role of HIV infection is less clear. Possible mechanisms such as immune activation, inflammation, coagulation disturbances, lipoprotein particle changes,15 antiretroviral toxicity,10 and direct or immune-mediated damage of endothelial and myocardial cells, are less well established and require validation.16
Ventricular filling abnormalities and diastolic dysfunction are the first manifestations of myocardial injury irrespective of the cause,17,18 and are widely reported in HIV-infected populations with and without antiretroviral treatment or without established cardiovascular disease.19
It is not known whether cardiac disease can be diagnosed in the early stages prior to established diastolic dysfunction. Most myocardial pathologic processes are usually regional before becoming widespread, and local ventricular mechanics can be accurately assessed using strain and strain rate (SR).
The aim of this study was to reveal abnormalities in cardiac function due to HIV infection using strain and strain rate analysis in infected subjects without cardiovascular disease.
MethodsPopulation and eligibilityThe enrolled population were HIV-infected individuals receiving routine care in the outpatient clinic. Only patients without cardiovascular disease and aged 25–55 years were recruited, to avoid possible confounding myocardial relaxation abnormalities.20 Both patients treated with antiretroviral drugs and untreated patients were included. Pregnant women, patients with diabetes, hypertension, dyslipidemia, significant cardiac valve disease, renal disease, liver disease, Centers for Disease Control and Prevention (CDC) class C patients, and those with fever, treated with interferon or corticoids, and unable to read and sign the informed consent were excluded.
Initial assessment included a clinical questionnaire, physical examination, blood pressure measurement and a 12-lead electrocardiogram.
The control group were 20 healthy hospital workers, frequency matched for gender and without cardiovascular risk factors, who volunteered to undergo an echocardiogram and a physical exam.
Our hospital's ethics committee approved the study protocol, and all patients provided written informed consent.
Laboratory assessmentAll patients underwent laboratory tests, including hemogram, serum electrolytes, blood glucose, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, viral load and CD4 count.
Detectable viral load was defined as more than 20 copies of HIV RNA.
EchocardiographyEchocardiographic examinations were performed using a Vivid 7 digital ultrasound system with a 1.5–5 MHz probe (GE Medical Systems, Horten, Norway) and analyzed in EchoPac (GE Healthcare). Three cardiac cycles were stored in cineloop format for offline analysis.
Left ventricular and left atrial dimensions were measured according to the recommendations of the American Society of Echocardiography.21 Left ventricular ejection fraction was calculated by the change in ventricular volume percentages between systole and diastole in 2- and 4-chamber apical view using the modified Simpson's method (mean of three measurements).21
Left ventricular filling was assessed by spectral pulsed Doppler, with the sample volume positioned over the flow immediately above the mitral leaflets. Peak early diastolic flow velocity (E), peak atrial contraction flow velocity (A) and the E/A ratio were obtained. Pulsed tissue Doppler was used to assess longitudinal motion of the mitral annulus. Particular care was taken to ensure that the ultrasound beam was aligned parallel to the direction of annular motion. Peak septal and lateral wall velocities of the systolic wave S (S’), E wave (E’) and A wave (A’) were determined and the mean E/E’ ratio was calculated.
Diastolic function was evaluated and graded as recommended by the guidelines. In preserved systolic function, a left atrial volume less than 34 ml/m2, lateral E’ ≥10 m/s and septal E’ ≥8 m/s confirmed that diastolic dynamics was normal. An atrial volume of more than 34 ml/m2, lateral E’ <10 m/s with septal E’ <8 m/s and mean E/E’ <8 classified the diastolic dysfunction as grade I. When mean E/E’ was >8 and <13 diastolic dysfunction was classified as grade II and as grade III when mean E/E’ was ≥13.22
Strain rate measurements from the basal segments were obtained by color tissue Doppler (>140 fps). The acquired velocity data allowed direct calculation of regional SR, and the basal segments were chosen because angle deviations could be easily avoided, making the assessment more reproducible. Left outflow tract and transmitral flows were acquired to determine the timing of cardiac cycle events. The angle of interrogation was kept as small as possible and aligned parallel to the region of interest, in 4- and 2-chamber and apical long-axis views. Systolic strain rate (SRS), early diastolic strain rate (SRE), and late diastolic strain rate (SRA) were assessed offline from basal segments of the six walls.23 The mean SRS, SRE, and SRA correspond to the mean of the six basal segments.
Longitudinal, radial and circumferential strain were obtained by 2D speckle tracking. The frame rate was reduced to 58–85 fps in order to improve tracking quality and the angle was not so critical as the technique is angle-independent. Longitudinal strain was assessed in apical view and circumferential and radial strain in parasternal short-axis view. Global longitudinal strain was calculated by the mean longitudinal strain of the six walls (basal, mid and apical segments) in apical view and mean circumferential and radial strain for the six mid left ventricular segments.
Images from 10 random patients were analyzed offline again and by a second blinded observer to assess intra- and inter-observer variability in strain and strain rate measurements.
Statistical analysisThe control group was only frequency matched for gender. Dichotomous data were expressed as number and percentage and compared with the chi-squared test. Numerical variables were expressed as mean and standard deviation and were analyzed using the Student's t test for independent samples. A multiple regression model was also applied.
Pearson's correlation analysis was used to evaluate the relationship between CD4 count, viral load, duration of infection and the various echocardiographic parameters.
In accordance with the CDC classification, the population was divided in three groups (CD4 <200 cells/mm3, 2003 and CD4 >500 cells/mm3). The echocardiographic findings were compared with one-way ANOVA and multivariate analysis was performed with MANOVA.
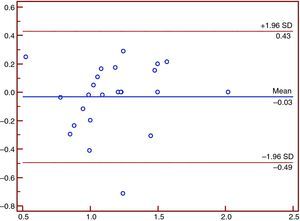
Intra- and inter-observer variability was tested by the Bland-Altman method,24 calculated as the absolute difference divided by the mean of the two observations.
A p value of less than 0.05 was considered statistically significant.
Analysis was performed using SPSS software, version 17.0.0 (SPSS, Inc., Ill., USA).
ResultsA total of 200 patients were screened in the outpatient clinic for study inclusion and 80 were potentially eligible. Three were excluded because of hypertension at the clinical exam, one had severe aortic stenosis, and 26 refused to provide written informed consent. Overall 50 patients were included, mean age 41±6 years, 64% male. All the electrocardiograms were normal.
Table 1 summarizes the characteristics of the HIV-infected population. One patient has been infected for 30 years, and another less than a year. There was a high prevalence of smokers (74%, 37 patients).
Characteristics of the HIV-infected study population.
| Population (n=50) | |
| Age (years) | 41±6 |
| Male gender | 32 (64%) |
| Caucasian | 48 (96%) |
| BMI (kg/m2) | 24±4.7 |
| Disease (years) | 10±5 |
| SBP (mmHg) | 122±12 |
| DBP (mmHg) | 78±10 |
| Blood glucose (mg/dl) | 97±13 |
| LDL cholesterol (mg/dl) | 115±28 |
| CD4 >500/mm3 | 27 (54%) |
| 2003 | 18 (36%) |
| CD4 <200/mm3 | 5 (10%) |
| Viral load >20 copies | 16 (32%) |
| NRTIs | 40 (80%) |
| NNRTIs | 10 (20%) |
| PIs | 34 (68%) |
| IIs | 2 (4%) |
| CCR5rAs | 1 (2%) |
| Under treatment | 43 (86%) |
| LA volume (ml/m2) | 26±6 |
| E wave (m/s) | 0.72±0.16 |
| A wave (m/s) | 0.57±0.17 |
| E/A ratio | 1.33±0.33 |
| Mean E’ (m/s) | 0.12±0.03 |
| Mean E/E’ | 5.7±1.8 |
| Mean S’ (m/s) | 0.09±0.02 |
| Ejection fraction (%) | 60±6 |
| LVEDV (ml/m2) | 55±9 |
| LVESV (ml/m2) | 23±5 |
| LV mass (g/m2) | 76±19 |
| LS (%) | −19.5±1.9 |
| RS (%) | 54±16 |
| CS (%) | −17.8±3.5 |
| SRS (s−1) | −1.1±0.3 |
| SRE (s−1) | 1.8±0.4 |
| SRA (s−1) | 1.2±0.3 |
BMI: body mass index; CCR5rAs: CCR5 receptor antagonists; CS: circumferential strain; DBP: diastolic blood pressure; IIs: integrase inhibitors; LA: left atrial; LS: global longitudinal strain; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; NNRTIs: non-nucleoside reverse transcriptase inhibitors; NRTIs: nucleotide reverse transcriptase inhibitors; PIs: protease inhibitors; RS: radial strain; SBP: systolic blood pressure; SRA: late diastolic strain rate; SRE: early diastolic strain rate; SRS: systolic strain rate.
Seven (14%) patients were not under active antiretroviral treatment: four did not fulfill clinical or laboratory criteria to start drugs, two were not compliant and the other presented an acute infection and started medication at the selection visit. Patients were taking a mean of 3.3 antiretroviral drugs, 66% were taking three, 16% four and 4% five drugs. Most were taking two antiretroviral classes, except two who were taking three classes.
CD4 count was 579±285 cells/mm3; maximum viral load was 463 000 copies and minimum was <20 copies. Fifteen patients (30%) had detectable viral load, of whom eight were under active treatment – six (75%) with ‘blips’ (intermittent episodes of detectable low-level HIV1), and two (25%) were frequently non-compliant.
Comparing the HIV population to controls, there were no differences in demographic and clinical data between groups except for diastolic blood pressure and smoking (37 smokers in the HIV group and none in the control group, p<0.0001) (Table 2).
Clinical characteristics of the HIV-infected population and the control group.
| Control group (n=20) | HIV-infected population (n=50) | p | |
| Age (years) | 39±7 | 41±6 | 0.2 |
| Male gender | 12 (60%) | 32 (64%) | 0.79 |
| Caucasian | 19 (95%) | 48 (96%) | 1 |
| SBP (mmHg) | 117±11 | 122±12 | 0.13 |
| DBP (mmHg) | 69±8 | 78±10 | 0.04 |
| BMI (kg/m2) | 26±5 | 24±5 | 0.18 |
Abbreviations as for Table 1.
As shown in Table 3, there were no differences in systolic or diastolic function parameters, except for peak E-wave velocity, which was higher in controls. One HIV patient had diastolic dysfunction classified as grade I (E/E’ <8).
Conventional echocardiographic parameters of the HIV-infected population and the control group.
| Control group (n=20) | HIV-infected population (n=50) | p | |
| LV mass (g/m2) | 75±22 | 76±19 | 0.8 |
| LA volume (ml/m2) | 25±5 | 26±6 | 0.5 |
| RA volume (cm2/m2) | 7.3±1.5 | 7.6±1.4 | 0.4 |
| E wave (m/s) | 0.84±0.16 | 0.72±0.17 | 0.01 |
| E/A ratio | 1.5±0.38 | 1.3±0.34 | 0.2 |
| Mean E’ (m/s) | 0.14±0.03 | 0.12±0.03 | 0.1 |
| Mean E/E’ | 6±1.3 | 6±1.8 | 0.4 |
| Ejection fraction (%) | 60±5 | 60±7 | 0.8 |
| Mean S’ (m/s) | 0.1±0.02 | 0.09±0.02 | 0.4 |
RA: right atrial. Other abbreviations as for Table 1.
The 2D strain software achieved acceptable tracking in 97% of cases in longitudinal strain, 98% in radial strain and 96% in circumferential strain.
Global longitudinal strain, mean radial and circumferential strain and mean SRS, SRE, and SRA are reported in Table 4. Global longitudinal strain and SRS and SRE in the HIV group, although in the normal range,23 were significantly lower than in the control group. When analyzed in a multiple regression model including clinical and echocardiographic parameters, only SRS (p=0.03, 95% CI 0.036:0.671) and SRE (p=0.001, 95% CI −0.599: −0.168) were different between groups.
Strain and strain rate parameters in the HIV-infected population and the control group.
| Control group (n=20) | HIV-infected population (n=50) | p | |
| Global LS (%) | −21±2 | −19.5±1.9 | 0.005 |
| Mean RS (%) | 48±15 | 54±16 | 0.18 |
| Mean CS (%) | −17±3 | −18±3.6 | 0.43 |
| SRS (s−1) | −1.3±0.28 | −1.1±0.28 | 0.02 |
| SRE (s−1) | 2.2±0.4 | 1.8±0.4 | <0.001 |
| SRA (s−1) | 1.2±0.3 | 1.2±0.3 | 0.87 |
Abbreviations as for Table 1.
Neither treatment (also tested for the different drug classes and drug combinations: nucleoside reverse transcriptase inhibitors and protease inhibitors or nucleoside reverse transcriptase inhibitors and non-nucleoside reverse transcriptase inhibitors) nor detectable viral load had any impact on echocardiographic parameters.
Circumferential strain was different between CD4 categories (CD4 <200: −16.97±3.8%; 200≤CD4 ≤500: −16.37±2.3%; CD4 >500: −19.98±3.9%; p=0.049) and was still different in multivariate analysis (p=0.04. 95% CI −4.59; −0.09).
The correlations obtained between disease duration and mean radial strain (c=−0.31, p=0.03), and immunologic state and mean circumferential strain (c=−0.4, p=0.005) were weak. No clinical or laboratory data correlated with global longitudinal strain (even when tested for the different coronary territories). The same was observed for the strain rate parameters.
Intra-observer and inter-observer variability and reproducibilityThese are shown in Figures 1–12.
Our study excluded patients with comorbidities, particularly established cardiovascular disease, that could cause myocardial damage and abnormal cardiac mechanics.
Although there was a high prevalence of smokers, the population was relatively healthy (systolic blood pressure 122±12 mmHg, blood glucose 97±13 mg/dl, LDL-cholesterol 115±28 mg/dl, 90% with CD4 count >200 cells/mm3, only 32% with detectable viral load, and 86% under antiretroviral treatment), a representative sample of HIV-infected populations in developed countries,25 potentially achieving a normal life expectancy.26
Previous echocardiographic studies in HIV-infected populations have focused on systolic and diastolic function assessed by 2D and spectral Doppler echocardiography, only the most recent17,19,27,28 using pulsed tissue Doppler, but none had classified and graded diastolic dysfunction according to the current guidelines.21
In our study there were no differences in systolic or diastolic function between groups when assessed with conventional echocardiography and pulsed tissue Doppler. Previous studies also showed no systolic abnormalities, but reported a high prevalence of diastolic dysfunction. This difference can be explained by comparing the studied populations. Our is a relatively healthy and young sample with no comorbidities, unlike the larger sample (689 patients) studied by Reinsch et al.19 in the HIV-Heart Study, who were older (44±10 years, p=0.04), 60% had dyslipidemia, 7% had diabetes and one third had hypertension. They used pulsed tissue Doppler and transmitral pulsed Doppler to assess diastolic dysfunction, which was present in 48% of the patients. Blood pressure, hyperlipidemia, diabetes, body mass index and age were different between groups with and without diastolic dysfunction, but infection-related conditions were similar (duration of infection, CD4 count, viral load and treatment). Two other smaller studies using pulsed tissue Doppler17,28,29 came to the same conclusions concerning a high prevalence of relaxation abnormalities, but again the classification and grading were not performed as recommended. In a sub-analysis30 of the SUN study with 659 patients, diastolic dysfunction correlated with high-sensitivity C-reactive protein and the presence of hypertension. Hsue et al.27 obtained similar results, the HIV-infected group having significantly more hypertension (26% vs. 6%), and in multivariate analysis, hypertension, age and HIV infection increased the risk for diastolic dysfunction (neither CD4 count, viral load, nor type of treatment increased the risk when adjusted for age and hypertension).
To summarize, our results, which are not in agreement with previous studies, can be explained by the fact that our HIV population were healthier compared with other, more heterogeneous HIV populations.
The population studied by Oliviero et al.31 was similar to ours in age and blood pressure but had a better metabolic status (blood glucose, p<0.001 and cholesterol, p=0.001), a shorter infection duration and were not under active antiretroviral therapy. They reported an abnormal relaxation pattern in 30% of patients, but compared with our results there were no differences in diastolic parameters (e’ 0.13±0.08 vs. 0.12±0.03, p=0.4; E/e’ 6.6±3.5 vs. 5.7±1.8, p=0.12).
Speckle tracking is a recent echocardiographic technology that analyzes motion by tracking natural acoustic reflections with relative angle independence, less noise interference and substantially lower intra-observer and inter-observer variability. Strain and strain rate are dimensionless measurements of deformation (degree of shortening or stretching of fibers). The degree of shearing increases toward the subendocardium. The subendocardial region that is assessed by longitudinal strain is the most vulnerable component of left ventricular mechanics and therefore most sensitive to the presence of myocardial disease. A transmural insult or progression of disease results in concomitant midmyocardial and subepicardial dysfunction, leading to a reduction in circumferential and radial strain.32
Our study was the first to assess strain and strain rate33 in a HIV-infected population without established cardiovascular disease or risk factors. Although the mean strain and strain rate obtained in the HIV group were in the normal range according to the latest ASE/EAE Consensus Statement,23 global longitudinal strain, SRS and SRE were statistically lower compared with the control group, revealing early abnormalities. It is important to note that diastolic dysfunction was not detected with conventional echocardiography but the presence of lower SRE reveals abnormalities in myocardial relaxation.
In the present study viral load had no impact on myocardial performance, probably because only 30% had detectable virus in blood samples, but circumferential strain differed according to immunological status (more than half of the patients had CD4 >500 cells/mm3), which is in agreement with the findings of previous studies that reported myocardial injury mediated by the non-myocyte pool, especially in patients with low CD4 count.34
Antiretroviral therapy is usually assumed to be toxic (particularly protease inhibitors associated with lipodystrophy leading to metabolic disorders including insulin resistance and dyslipidemia15,34,35), but in our sample the drugs had no perceptible impact when studied individually or in combination, probably because of the small number of patients enrolled.
Although we believe our data are relevant, not only because they were obtained with strain and strain rate methodology, but also because our population was healthy, we do acknowledge some limitations, including the small number of participants, the fact that it was an observational study, and the absence of biomarker assessment.
In conclusion, diastolic dysfunction assessed as recommended by the latest guidelines is not prevalent in a relatively healthy HIV-infected population without cardiovascular disease or risk factors. Although strain and strain rate are able to detect the first signs of myocardial mechanics abnormalities, because these techniques are still in the research stage, we consider it premature to recommend routine screening echocardiograms in HIV-infected adults without cardiovascular disease, mostly because the prognostic impact of these findings is unknown.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Mendes L, Silva D, Miranda C, Sá J, Duque L, Duarte N, et al. Impacto da infeção do vírus da imunodeficiência humana na deformação miocárdica 2013. Rev Port Cardiol. 2014;33:501–509.