Spontaneous coronary artery dissection is a rare cause of acute coronary events or sudden cardiac death. The clinical presentation is highly variable and prognosis varies widely, depending mainly on how rapidly it is diagnosed. Prompt treatment is also essential, and includes medical management, percutaneous coronary intervention and surgical revascularization.
We describe the case of a young woman presenting with spontaneous coronary artery dissection of the left main coronary artery, first diagnosed as coronary thrombus, who underwent successful percutaneous coronary stenting. This report highlights the need to include spontaneous coronary artery dissection in differential diagnosis of chest pain in young women and that distinguishing between coronary thrombus and coronary artery dissection is not always straightforward. To our knowledge this is the fourth case of left main stenting in a patient with spontaneous coronary artery dissection described in the literature.
As disseções coronárias espontâneas são uma causa rara de eventos coronários agudos ou de morte súbita cardíaca. A sua apresentação clínica e prognóstico são altamente variáveis e dependem, principalmente, da rapidez com que o diagnóstico é feito. O tratamento imediato é também essencial, e inclui abordagem médica, intervenção coronária percutânea e revascularização cirúrgica.
Descrevemos o caso de uma mulher jovem admitida com disseção espontânea do tronco comum da artéria coronária esquerda, primeiramente diagnosticada como trombo intracoronário. A paciente foi submetida a angioplastia percutânea mediante implantação de stent, com bom resultado angiográfico final. Este caso clínico enfatiza que a disseção coronária espontânea deve ser incluída no diagnóstico diferencial de dor torácica em mulheres jovens e, ainda, que a distinção entre trombo coronário e disseção coronária pode não ser simples. Este é o quarto caso de disseção do tronco comum tratado percutaneamente, mediante implantação de stent, que encontramos descrito na literatura.
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary events or sudden cardiac death.1,2 It often affects young women and usually involves a single coronary artery, mainly the left anterior descending (LAD) artery.1–4 SCAD has traditionally been observed in three groups of patients: those presenting with significant coronary atherosclerotic disease, women in the peripartum and early post-partum period or using oral contraceptives, and cases without obvious associated causal factors, termed idiopathic.5,6 The clinical presentation is highly variable and depends on the location, extent and severity of SCAD.1 Prognosis varies widely, but is generally dismal in the absence of prompt recognition and treatment, when the clinical presentation is sudden cardiac death, and in peripartum women.1,3 Treatment strategies include medical management, percutaneous coronary intervention (PCI) and surgical revascularization, the latter procedure being particularly indicated in cases of left main dissection, multivessel involvement and failed PCI procedures.1,7
We report the case of a young woman who presented with SCAD of the left main and underwent successful PCI.
Case reportA previously healthy 36-year-old woman presented to our institution, transferred from another center, with a diagnosis of non-ST elevation myocardial infarction, in cardiogenic shock (Killip class IV), for urgent coronary artery bypass graft (CABG) surgery. Her only cardiovascular risk factor was tobacco use and she had been on oral contraceptives for the last few years.
Previously that day, she had been admitted to another center for prolonged chest pain. Her physical examination was normal. The ECG demonstrated diffuse ST-segment depression, maximum 2 mm in V4-5 and I, and showed 1.5-mm ST-segment elevation in aVR. She was given 250 mg aspirin and 300 mg clopidogrel and treated with anti-ischemic drugs. Transthoracic echocardiogram (TTE) showed mild to moderate left ventricular (LV) systolic dysfunction, posterolateral akinesia, and moderate mitral regurgitation (MR) due to posterior leaflet restriction.
Due to recurrence of chest pain she underwent coronary angiography, which showed 50% distal left main coronary artery (LMCA) stenosis, 90% ostial LAD stenosis with an image suggestive of thrombus, occlusion of the left circumflex artery (LCx) at its origin and an angiographically normal right coronary artery. During catheterization, she became progressively unstable with severe hypotension and sustained chest pain. An intra-aortic balloon pump (IBP) was inserted and dopamine was initiated. The patient was transferred to our center for emergent CABG.
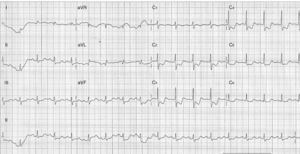
On admission, the patient was in cardiogenic shock (BP 70/50 mmHg), despite IBP and vasopressor support (VPS), with sustained and severe chest pain. The ECG showed accentuation of the previous alterations (Figure 1).
In view of the patient's unstable clinical condition, the need for immediate revascularization was balanced against the potential delay while awaiting preparation for bypass surgery. At a multidisciplinary meeting, it was therefore decided to attempt a percutaneous approach as first-line therapy.
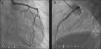
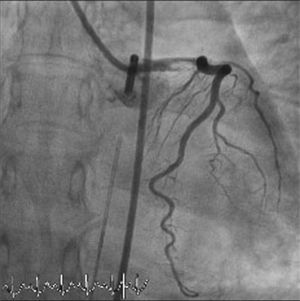
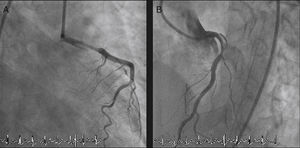
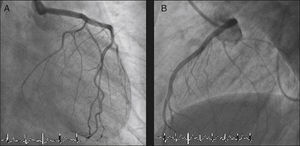
The LMCA was engaged with a 7-Fr JL4 guiding catheter (Cordis Corp., Miami Lakes, FL) (Figure 2; Video 1), and a 0.014-inch Runthrough guidewire (Terumo Medical Corp., Somerset, NJ) was passed to the distal LAD. An attempt was made to aspirate thrombotic material using an Export AP aspiration catheter (Medtronic Corp., Minneapolis), but with no success and no change in the angiographic appearance of the lesion. Neither the LCx nor its emergence from LMCA were visible, and we were unable to pass a guidewire through it. A more detailed evaluation of the lesions in several different projections showed an image suggestive of LMCA dissection (Figure 3A and B; Video 2A and B). Intravascular ultrasound was considered but was not used because of the patient's hemodynamic instability and the certainty of the diagnosis. Subsequently, the LMCA was predilated using a 3.0 mm × 15 mm balloon (TREK, Abbott Vascular, Abbott Park, IL) followed by the implantation of a drug-eluting stent (4.0 mm × 23 mm Xience Prime, Abbott Vascular, Abbott Park, IL), with a good angiographic result. After stenting, the LCx became visible and both the LAD and LCx presented no angiographic lesions (Figure 4A and B; Video 3A and B).
The patient had a favorable evolution, with IBP and VPS withdrawn within 48 hours and with no complications. Her serum troponin I peaked at 43.2 ng/ml and TTE showed mild LV systolic dysfunction and mild to moderate MR. Given her positive clinical course, on day four she was transferred back to the original hospital.
DiscussionSpontaneous coronary artery dissection (SCAD) is a rare and under-recognized cause of acute coronary events or sudden cardiac death.1,2 Its incidence ranges from 0.07 to 1.1% in angiographic series.1,2,4,8 It often affects young women,1–4 with a mean age at presentation of 30–45 years; more than 70% of cases are women (approximately 30% in the peripartum period)1,2.
Dissection of the LAD alone is most common (60–75% of cases),1,2,4–6,8 with multivessel and LMCA involvement in 20% and 12% of cases, respectively.1
SCAD has traditionally been observed in three groups of patients: those presenting with significant coronary atherosclerotic disease, women in the peripartum and early post-partum period, and patients without obvious associated causal factors, termed idiopathic.5,6 SCAD has also been associated with various other conditions including connective tissue disorders, vasculitis, strenuous exercise, prolonged sneezing, use of cocaine, cyclosporine and, as in our case, oral contraceptives.1,2,4–6 Although the precise etiology and pathogenesis of SCAD remain unclear, all these conditions are associated with weakening of connective tissue and/or vascular inflammation.
The clinical profile of SCAD also varies according to gender, with women being characterized by more frequent involvement of the LAD, in the absence of coronary atherosclerosis and traditional cardiovascular risk factors, and an increased risk of occurrence during peripartum and under estrogen treatment. In men presentation occurs later in life, the right coronary artery is more often affected and there is often coexisting atherosclerotic coronary disease and cardiovascular risk factors.2,4
The clinical presentation is highly variable and depends on the location, extent and severity of SCAD.1 The clinical spectrum includes unstable angina, acute myocardial infarction, heart failure, cardiogenic shock, cardiac tamponade, ventricular arrhythmias and sudden cardiac death.1,3 Rarely, it can be asymptomatic and an incidental finding on coronary angiography.1
Treatment strategies include medical management, PCI and surgical revascularization, and the choice of initial management depends essentially on the location and extension of the dissection, its functional repercussions, and the patient's clinical status. If there is no evidence of ongoing ischemia or hemodynamic instability, in cases of single-vessel dissection not affecting the LMCA with TIMI 3 flow, SCAD can probably be managed successfully with a conservative approach.1,6,7,9,10 Medical management includes antithrombotic therapy with heparin or low molecular weight heparin, aspirin, clopidogrel and glycoprotein IIb/IIIa inhibitors, and anti-ischemic therapy with beta-blockers and nitrates. However, it should be borne in mind that while potent antithrombotic therapy decreases thrombus formation in the false lumen, enhancing blood flow in the true lumen, it can also increase bleeding into the false lumen, causing extension of the dissection. This is especially true for fibrinolytics and their use should generally be avoided.1,3,5 In single-vessel dissection not involving the LMCA, with persistent impairment of blood flow and signs of ongoing ischemia, PCI with stenting is the procedure of choice.1,6,7 Intravascular ultrasound and optical coherence tomography may be of value in identification of the true lumen, accurate guidewire placement and appropriate stent choice and deployment.7,11,12 The use of drug-eluting stents (DES) in SCAD is questionable. While the frequent need for long stented segments in these cases may justify the preferential use of DES, on the other hand, these stents may delay vessel wall healing. The choice between bare-metal stents and DES remains subject of disagreement.2,7 CABG is usually reserved for patients with LMCA dissection, multivessel involvement and failed PCI.1,7 Nevertheless, in selected cases LMCA and multivessel dissections may be treated by stenting as well,5–7 although this is not a common approach.
Prognosis varies widely, but is generally dismal in the absence of prompt recognition and treatment, when the clinical presentation is sudden cardiac death, and in peripartum women.1,3 In earlier studies, mortality was approximately 50%, with 50% recurrence at two months.13 In-hospital mortality is now relatively low, with a mean rate of 3%.1,7 Those who survive the acute phase have a good long-term prognosis, with very low recurrence of dissection and a long-term survival of over 95%,14,15 with the strongest predictors of death being female gender and absence of early treatment.14
The case reported was a typical SCAD patient – a young woman, under oral contraceptive therapy and a smoker. Even so, the definite diagnosis was not clear-cut. Percutaneous treatment of LMCA dissection has not often been reported in the literature (to our knowledge this is the fourth case described of left main stenting in a patient with SCAD5,16). In our case, given the rapid deterioration of the patient, with hemodynamic instability, a percutaneous approach seemed the most expeditious therapy.
This case highlights the need to consider SCAD in the differential diagnosis of precordial pain in young women, especially in the peripartum period and in those using oral contraceptives, without classic cardiovascular risk factors. It should be borne in mind that differential diagnosis between thrombus and SCAD is not always straightforward on angiography, and visualization in different projections and complementary techniques, such as intravascular ultrasound and optical coherence tomography, are essential for correct diagnosis. In certain clinical scenarios, a percutaneous approach may be considered in spontaneous LMCA dissection.
Ethical disclosuresRight to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
The following are the supplementary data to this article: