Metaiodobenzylguanidine (MIBG) is a false neurotransmitter noradrenaline analogue that is taken up by the ‘uptake 1’ transporter mechanism in the cell membrane of presynaptic adrenergic neurons and accumulates in catecholamine storage vesicles. Since it is practically unmetabolized, it can be labeled with a radioisotope (iodine-123) in scintigraphic exams to noninvasively assess the functional status of the sympathetic innervation of organs with a significant adrenergic component, including the heart. Studies of its application in nuclear cardiology appear to confirm its value in the assessment of conditions such as coronary artery disease, heart failure, arrhythmias and sudden death.
Heart failure is a global problem, with an estimated prevalence of 2% in developed countries. Sudden cardiac death is the main cause of its high mortality. The autonomic nervous system dysfunction, including sympathetic hyperactivity, that accompanies chronic heart failure is associated with progressive myocardial remodeling, declining left ventricular function and worsening symptoms, and contributes to the development of ventricular arrhythmias and sudden death.
Since 123I-MIBG cardiac scintigraphy can detect changes in the cardiac adrenergic system, there is considerable interest in its role in obtaining diagnostic and prognostic information in patients with heart failure.
In this article we present a literature review on the use of 123I-MIBG scintigraphy for risk stratification of sudden death in patients with heart failure.
A metaiodobenzilguanidina (MIBG) é um falso neurotransmissor análogo da noradrenalina, captada essencialmente por um mecanismo de transporte de tipo 1 na membrana celular dos neurónios adrenérgicos pré-sinápticos, acumulando-se em grânulos de armazenamento de catecolaminas. Como praticamente não é metabolizada, a sua marcação com um radioisótopo (iodo-123) permite, através de imagens cintigráficas, avaliar de forma não invasiva o status funcional da inervação simpática de órgãos com importante componente adrenérgico, incluindo o coração. A sua aplicabilidade em cardiologia nuclear tem vindo a ser estudada e parece revelar importância na avaliação de patologias como a doença arterial coronária, insuficiência cardíaca, arritmias e morte súbita.
A insuficiência cardíaca é um problema à escala global, com uma prevalência estimada nos países desenvolvidos de 2%. Apresenta uma mortalidade elevada, sendo a morte súbita cardíaca a principal causa. A disfunção do sistema nervoso autónomo, nomeadamente a hiperatividade simpática, que acompanha a insuficiência cardíaca crónica, relaciona-se com a remodelação progressiva do miocárdio, o declínio da função ventricular esquerda e o agravamento dos sintomas, participando no desenvolvimento de arritmias ventriculares e morte súbita.
Dado que a cintigrafia cardíaca com 123I-MIBG permite a identificação de alterações do sistema adrenérgico cardíaco, questiona-se o seu papel na obtenção de informação diagnóstica e prognóstica em doentes com insuficiência cardíaca.
Pelo interesse e a atualidade do assunto, pareceu-nos oportuno rever os dados publicados sobre a utilização da cintigrafia com 123I-MIBG na estratificação do risco de morte súbita em pacientes com insuficiência cardíaca.
autonomic nervous system
brain natriuretic peptide
implantable cardioverter-defibrillator
left ventricular ejection fraction
heart/mediastinum ratio
metaiodobenzylguanidine
noradrenaline
New York Heart Association
parasympathetic nervous system
sudden cardiac death
sympathetic nervous system
single-photon emission computed tomography
ventricular fibrillation
ventricular tachycardia
washout rate
Heart failure (HF) has an estimated global prevalence of 2–3%.1 In Portugal, its overall prevalence is 4.3%, increasing with age (estimated at 16.14% in those aged over 80).2 Despite advances in treatment, the number of patients with HF continues to rise in developed countries with ageing populations.
Systolic HF develops following myocardial damage; it is accompanied by a decline in cardiac function, which activates compensatory mechanisms designed to preserve cardiac homeostasis that initially maintain heart rate, blood pressure and cardiac output, keeping the patient asymptomatic.3,4 The most important elements involved are the renin–angiotensin–aldosterone axis, the sympathetic nervous system (SNS) and cytokines, chronic activation of which lead to changes in cardiac structure and performance as the disease progresses,4 including hypertrophy and myocyte apoptosis, fibroblastic proliferation and interstitial collagen accumulation. The result is myocardial remodeling and contractile dysfunction and hence reduced left ventricular ejection fraction (LVEF),3 as well as increased susceptibility to arrhythmias and sudden cardiac death (SCD).5 In diastolic HF, by contrast, LVEF is preserved.1,3,6
SCD is one of the main causes of mortality in HF7 (30–50%8), mainly due to ventricular tachycardia (VT), ventricular fibrillation (VF) and bradycardia. The autonomic nervous system (ANS) plays a central role in these arrhythmias9,10; the electrophysiological and potentially arrhythmogenic effects of catecholamines have been shown to be one of the main causes of VT and SCD in patients with autonomic dysfunction and sympathetic hyperactivity.11
The pro-arrhythmic effects of HF, which are related to changes in intracellular calcium concentrations and ANS tone, predispose to tachyarrhythmias and bradyarrhythmias that can result in cardiac arrest and SCD.12 The most common electrical activation sequence is VT degenerating into VF; this is most often seen in dilated cardiomyopathy and ventricular dysfunction with reduced LVEF.13
The decision to use an implantable cardioverter-defibrillator (ICD) is based on the patient's LVEF and New York Heart Association (NYHA) functional class, which are the parameters most commonly used to determine risk for SCD.14 This decision is a complex one: not all patients with the same LVEF have the same risk for arrhythmic death, while patients with very low LVEF have a greater risk for non-arrhythmic death and thus benefit less from an ICD.12 Only a third of patients who die suddenly meet LVEF-based criteria for prophylactic ICD placement under the current guidelines,15 and thus two-thirds of patients who could benefit from the device succumb to SCD, since their LVEF values do not meet the criteria for ICD implantation.10 This means that LVEF is not an adequate marker for SCD, which explains the increased prevalence of SCD despite use of ICDs. There is thus a need for more effective and specific markers for SCD risk stratification to improve selection of candidates for ICD implantation. Scintigraphy with metaiodobenzylguanidine (MIBG) tagged with the radioisotope iodine-123 (123I-MIBG) has been the subject of considerable research for this purpose. MIBG is produced by the chemical modification of guanethidine, a false neurotransmitter noradrenaline (NA) analogue16 that is taken up by presynaptic adrenergic neurons through the ‘uptake 1’ mechanism, an active, temperature-dependent process that is mediated by the transporter protein, sodium and energy.16,17 After uptake MIBG is accumulates in catecholamine storage vesicles,17 and since it is not metabolized by monoamine oxidase or catechol-o-methyltransferase, its cytoplasmic concentration remains higher than that of NA.16 It is thus possible to combine MIBG with a radioisotope and to record scintigraphic images of MIBG uptake and washout, which provide information on cardiac sympathetic function and the integrity of adrenergic neurons.18123I-MIBG as visualized by a gamma camera is used to obtain minimally invasive images, planar or tomographic (single-photon emission computed tomography, SPECT), from which the two fundamental semi-quantitative parameters can be calculated: the heart/mediastinum ratio (H/M), and the myocardial washout rate (WR).19 These parameters are directly related to release and reuptake of NA by nerve endings; local NA concentrations in the synaptic cleft increase with increased WR and decreased H/M.18
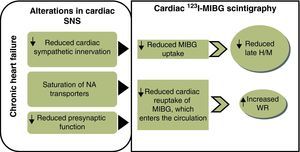
Changes in cardiac sympathetic function and 123I-MIBG imagingIn HF there is SNS hyperactivity (up to 50 times more active than normal), which is a compensatory mechanism for the cardiac dysfunction associated with the syndrome.20,21 SNS activation is a hemodynamic defense mechanism that is effective in the initial stages of HF but has long-term deleterious consequences, including structural remodeling associated with myocyte apoptosis, fibrosis and ventricular dilatation, as well as increased risk of electrical instability and progressive functional deterioration.2 Heightened adrenergic activity is accompanied by increased NA concentrations, which in the early stages of HF is only in the heart, but as the disease progresses excessive levels of NA in the synaptic cleft exhaust the binding and reuptake capacity of the receptors, leading to outflow of NA from the heart to peripheral tissues and to increased plasma concentrations.21
Long-term adrenergic hyperactivity also results in desensitization and significantly reduced expression of transporter proteins, raising synaptic NA levels22 and depleting NA storage vesicles,23 leading to a decline in myocardial sympathetic innervation.20 As myocardial catecholamine levels fall, selective desensitization of beta-adrenergic receptors occurs,23 which alters the ratio of beta-1 to beta-2 adrenoreceptors, reducing the density of the former and increasing that of beta-2 and beta-3 receptors 2–3 fold.24
These changes can result in fibroblastic hyperplasia, cardiomyocyte apoptosis and electrical instability.24 Excess of myocardial catecholamines alters electrophysiological mechanisms, changing membrane potentials and electrical conduction patterns and predisposing to reentrant mechanisms that modify cardiac excitability and predispose to fatal tachyarrhythmias.25
A study by Rocca et al.26 on the association of cardiac sympathetic hyperactivity with SCD and with worsening heart failure in patients with systolic dysfunction concluded that heightened adrenergic activity is associated with worse prognosis and is a risk factor for SCD. Increased cardiac NA stores, arterial NA concentration and whole body NA spillover are also suggested as strong risk factors for SCD.26
Cardiac 123I-MIBG scintigraphy provides information on neuronal integrity and sympathetic function in the heart through analysis of uptake and washout of the radioisotope in images taken up to 3–4 hours after intravenous injection. Early images (15–30 min after injection) show interstitial uptake, while late images assess neuronal uptake and are thus more often used.27 Images are interpreted by analyzing overall uptake of 123I-MIBG in planar images and comparing early and late images to determine washout, on the basis of which the two semi-quantitative indices, H/M and WR, are calculated.
H/M obtained from late images is the standard measure of 123I-MIBG uptake and reflects the distribution of cardiac sympathetic nerve endings.19 It is calculated by determining the mean pixel count on planar images of a region of interest on the heart (the left ventricular wall) and of another in the upper third of the mediastinum, and dividing the cardiac by the mediastinal count.19 Normal values range from 1.8 to 2.8 (mean 2.2±0.3), meaning that MIBG is taken up mainly in the myocardium in normal hearts; thus, a lower H/M reflects reduced myocardial uptake and hence lower density of cardiac adrenergic receptors. The early H/M ratio probably reflects the integrity of presynaptic nerve terminals, while the late H/M ratio combines neuronal function from uptake to release of 123I-MIBG through the storage vesicles at the nerve terminals.28 Myocardial washout, expressed as a percentage, is the rate at which pixel counts in the cardiac region of interest fall between early and late images, and thus represents the ability of the myocardium to retain MIBG, which depends on neuronal integrity and myocardial sympathetic tone.19
Various studies have demonstrated the ability of 123I-MIBG scintigraphy to detect the alterations in cardiac adrenergic function that accompany the pathophysiological processes of HF. These alterations, manifested by variations in H/M and WR (Figure 1), have been shown to be valuable in prognostic7 and risk7,18 stratification. As well as its role in assessing cardiac function, 123I-MIBG scintigraphy is also able to evaluate the effects of therapeutic interventions16 and to help define new treatment strategies.
Prognostic value of 123I-MIBG scintigraphy in heart failureThe first demonstration of the prognostic value of 123I-MIBG scintigraphy was by Merlet et al. in 1992, in a study of 90 HF patients with LVEF <45% and in New York Heart Association (NYHA) functional classes II-IV.29 They concluded that 123I-MIBG uptake as expressed by H/M correlated with life expectancy of patients with dilated or ischemic cardiomyopathy, H/M <1.20 indicating worse prognosis and lower survival. These observations suggested that the prognostic value of cardiac123I-MIBG scintigraphy was greater than that of LVEF.29
Several more recent studies have confirmed the value of scintigraphic parameters as prognostic and risk indicators in HF. A meta-analysis of 18 studies by Verberne et al. in 2008 showed that 123I-MIBG scintigraphic parameters were promising prognostic markers in patients with systolic HF, with low late H/M being associated with a greater incidence of cardiac events although not with cardiac death, and higher WR being linked to more cardiac events including cardiac death.30
A 2009 study by Tamaki et al. comparing the prognostic value of scintigraphic parameters with that of other markers demonstrated that WR is the strongest predictor of SCD in patients with mild to moderate HF (Table 1).31
Principal clinical trials analyzing 123I-MIBG scintigraphic parameters and risk of sudden death in heart failure patients.
| Number of patients | LVEF | Follow-up | HR (95% CI) (cox univariate analysis) | ||
| Late H/M | WR | ||||
| Kioka et al. (2007)40 | 97 | <40% | 65 months | 0.065 (0.010–0.426) | 1.057 (1.023–1.091) |
| Tamaki et al. (2009)31 | 106 | <40% | 65 ± 31 months | 0.131 (0.034–0.511) | 1.045 (1.017–1.074) |
| Kuramoto et al. (2010)36 | 106 | <40% | 6.8 ± 3.5 years | 0.089 (0.019–0.412) | 1.052 (1.022–1.082) |
CI: confidence interval; H/M: heart/mediastinum ratio; HR: hazard ratio; LVEF: left ventricular ejection fraction; WR: washout rate.
In 2010, a prospective study of 64 HF patients with LVEF <45% confirmed the prognostic value of H/M in chronic HF due to ischemia or idiopathic dilated cardiomyopathy.32 The authors used an H/M cutoff of 1.68, lower values being associated with significantly higher mortality (15 deaths out of 29 patients) than in those with H/M >1.68 (four deaths out of 36 patients). As well as showing that H/M is an independent marker of long-term prognosis in HF, the authors concluded that neither NYHA class nor resting LVEF correlates with prognosis. They also found that a combination of different markers (NYHA class, LVEF, plasma brain natriuretic peptide [BNP] levels, and late H/M) had greater prognostic value than any of these in isolation.32 The long-term (10-year follow-up) prognostic value of 123I-MIBG scintigraphy compared with conventional functional parameters was also studied by Momose et al. in 86 patients with dilated cardiomyopathy.33 The authors concluded that the main indicators of poor prognosis were LVEF <30%, WR >50% and late H/M <1.45; WR was the only independent risk marker of sudden death.33 They thus also showed that a combination of variables has greater prognostic value than scintigraphic parameters in isolation.
The first large prospective multicenter study was published in 2010, the ADMIRE-HF trial, which included 961 HF patients in NYHA class II or III. The trial's conclusions confirmed the value of 123I-MIBG scintigraphy for assessing adrenergic activity and prognosis in ischemic and non-ischemic HF.34 Primary analysis demonstrated that late H/M (determined four hours after tracer injection, in planar images) helps establish the probability of cardiac death (SCD or from HF). The cutoff established in this study was 1.6: patients with H/M ≥1.6 had a two-year risk of cardiac death of 1.8% as opposed to 11.2% in those with late H/M <1.6. Mortality was around 20% in patients with H/M <1.2. Late H/M provided additional information to that of plasma BNP and LVEF for identifying patients at greater risk of cardiac events (progression of HF, potentially fatal arrhythmia and cardiac death). The other semi-quantitative scintigraphic parameters, early H/M and WR, were also associated with risk for cardiac events, but late H/M was the only one with statistical significance and independent prognostic value.34
A recent meta-analysis of studies in Japan assessing the prognostic value of MIBG scintigraphic parameters concluded that the low late H/M and high WR seen in chronic HF are associated with fatal events.35 There was agreement among all the studies analyzed that late H/M was the best prognostic marker in chronic HF, corroborating the findings referred to above.35
The Seattle Heart Failure Model has been validated for estimation of total mortality in chronic HF. A 2011 study by Kuramoto et al. investigated the ability of 123I-MIBG scintigraphy to provide prognostic information additional to the Seattle model. The Seattle score was calculated and planar 123I-MIBG scintigraphic images were obtained in 106 HF patients in NYHA class I–III and LVEF <40% with an endpoint of cardiac death (SCD, death from HF and cardiac death from other causes such as myocardial infarction) in a mean follow-up of 6.8 years. Cox multivariate analysis showed that the Seattle score and MIBG WR were independent predictors of cardiac death. The study also showed that WR provides additional long-term prognostic information to the Seattle score and is the only independent predictor of SCD. The combination of the two parameters has greater sensitivity in identifying patients with chronic HF at higher risk of death.36
The data on the prognostic value of cardiac 123I-MIBG scintigraphy presented so far relate to HF with reduced LVEF, but there is also the question of its usefulness in assessing HF with preserved LVEF. This was addressed by Katoh et al. in a 2010 prospective study of 117 HF patients with LVEF ≥50%, with the endpoints of cardiac death (defined as death from worsening HF or SCD) and worsening HF requiring readmission, in a mean follow-up of 1025 days.37 The authors concluded that, as in HF with LVEF <50%, cardiac sympathetic hyperactivity is also involved in the pathogenesis of HF with preserved LVEF and that 123I-MIBG scintigraphy should therefore be useful in determining prognosis in these patients. The scintigraphic parameter with the greatest prognostic value was WR, which was the only independent predictor of cardiac events. Although their LVEF is within normal limits, these patients have a poor prognosis; 42 (36%) of the 117 patients in the study suffered cardiac events in the three-year period.37123I-MIBG scintigraphy thus appears to be of value in assessing the prognosis of HF patients with preserved LVEF. Further studies are needed to clarify its precise role in assessing the prognosis of patients with HF.
123I-MIBG scintigraphy in assessment of HF patients prior to implantation of a cardioverter-defibrillatorChanges in the cardiac sympathetic nervous system have been studied as possible risk markers for SCD and can thus help in selecting candidates for ICD implantation. Various studies have demonstrated the ability of cardiac 123I-MIBG scintigraphy to identify patients at greater risk for spontaneous ventricular tachyarrhythmias,11 appropriate ICD shocks38,39 and SCD (Table 1).9,31,34,36,40
The first study to evaluate the usefulness of 123I-MIBG scintigraphy as a predictor of sudden death in HF was by Kioka et al. in 2007,40 in a prospective study of 97 patients with mild to moderate HF (LVEF <40%). Scintigraphic parameters were associated with sudden death, but only WR was an independent predictor, in contrast to H/M, LVEF and NYHA class. More recent studies have confirmed these findings and shown WR to be a strong predictor of ventricular arrhythmias9 and SCD.31,33,36
Sudden death is by its nature difficult to study, being unexpected and frequently of unknown cause. One way to avoid this problem is to design trials on potentially fatal arrhythmias in which the subjects have ICDs, and many such studies have set out to determine the value of cardiac scintigraphy in the assessment of risk for ventricular tachyarrhythmias9 and SCD.38,39,41
In a prospective study published in 2008 of 54 patients with ICDs, Nagahara et al. observed that H/M predicted ICD shocks and risk of SCD, independently of LVEF and BNP, and that cardiac scintigraphy was able to distinguish patients at high or low risk for SCD and ventricular tachyarrhythmias resulting in appropriate shocks, but failed to identify those at intermediate risk. However, combining scintigraphic parameters with other clinical parameters, including BNP and LVEF, could more effectively evaluate the need for ICD treatment in patients at intermediate risk. Three variables were defined as able to identify patients at greater risk for SCD who would benefit most from an ICD: H/M less than 1.95 with a plasma BNP level of more than 187 pg/ml or an LVEF of less than 50%.38
Further evidence of the value of late H/M as a risk marker for SCD in HF was provided by Jacobson et al. in the ADMIRE-HF trial, published in 2010.34 In this multicenter trial cardiac events, including nonfatal arrhythmic events and sudden death, were more frequent in patients with H/M <1.60.
Arrhythmic events and sudden death in HF patients have been linked not only to impaired myocardial sympathetic function but also to alterations in myocardial perfusion and innervation. Regional variations in perfusion and sympathetic innervation have been implicated in the genesis of arrhythmias, particularly in patients with a history of ischemic heart disease.27 A prospective study by Nishisato et al. on the prognostic value of cardiac 123I-MIBG scintigraphy in association with myocardial perfusion studies confirmed a correlation between impaired myocardial innervation and perfusion and arrhythmic events and sudden death.41 This suggests that a combination of techniques to assess perfusion and adrenergic innervation can help identify patients at high risk.
The prognostic value of cardiac 123I-MIBG scintigraphic parameters derived from planar images has been amply demonstrated. By contrast, the predictive value of SPECT imaging has rarely been investigated in the assessment of cardiac sympathetic innervation. Boogers et al. analyzed the use of 123I-MIBG scintigraphy in predicting ventricular arrhythmias in 116 patients referred for an ICD, in whom planar and SPECT images were obtained before device implantation.39 Myocardial sympathetic denervation observed in SPECT images predicted ventricular arrhythmias, improving risk stratification for arrhythmic death. This study thus suggests a new prognostic marker: myocardial denervation as demonstrated by the 123I-MIBG SPECT defect score.
ConclusionsMortality associated with HF, particularly sudden death, remains high despite advances in treatment and in preventive measures.
Cardiac 123I-MIBG scintigraphy is able to reveal alterations in the cardiac SNS, and thus contributes to the assessment of HF patients and to selection of candidates for ICD implantation. Clinical application of this technique will depend on the results of multicenter studies with appropriate population sizes and follow-up periods, and should also include HF patients with preserved LVEF. However, it is also important to bear in mind the fact that the exam is costly and technically demanding, which is a significant obstacle to its more widespread use.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martins da Silva MI, et al. Utilização da cintigrafia com iodo-123-metaiodobenzilguanidina na estratificação do risco de morte súbita na insuficiência cardíaca. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.11.003.





 MIBG: metaiodobenzylguanidine; NA: noradrenaline;
MIBG: metaiodobenzylguanidine; NA: noradrenaline; 

