Spontaneous coronary artery dissection is a rare cause of acute coronary syndrome that typically occurs in relatively young patients without classical cardiovascular risk factors for coronary artery disease. The etiology appears to be multifactorial and optimal management is not clearly established, so the treatment strategy is often selected based on clinical presentation and coronary anatomy. We present two cases of spontaneous coronary artery dissection with different initial approaches, highlighting the importance of a case-by-case assessment.
A disseção coronária espontânea é uma causa rara de síndrome coronária aguda e ocorre tipicamente em indivíduos jovens sem os fatores de risco cardiovasculares clássicos de doença arterial coronária. Apresenta etiologia multifatorial e tratamento não definido, pelo que a sua abordagem deve ser feita de forma selecionada baseada na apresentação clinica e anatomia coronária. Apresentam-se dois casos clínicos de disseção coronária espontânea com diferente abordagem inicial, que espelham a importância da avaliação individualizada de acordo com a apresentação clínica.
Spontaneous coronary artery dissection (SCAD) is a rare condition, and its etiology, pathophysiology and treatment are not established. Various factors, including clinical presentation, hemodynamic status, and coronary anatomy, influence the treatment strategy, which in the absence of solid evidence should be approached on a case-by-case basis.
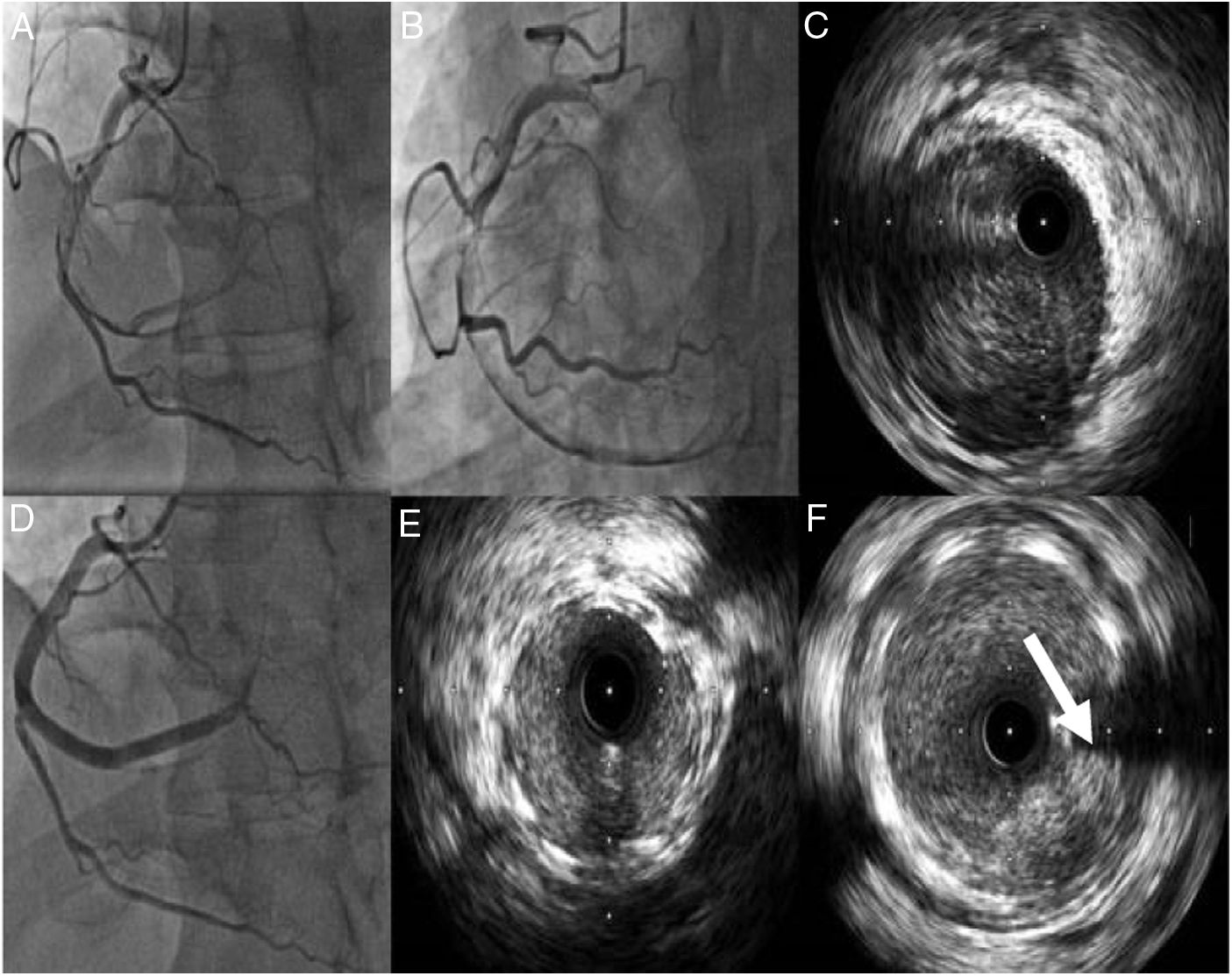
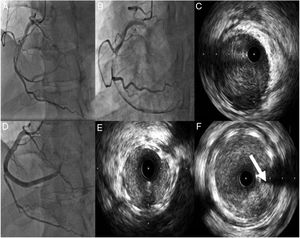
Case 1A 42-year-old man, previously healthy and an active sportsman, with no known cardiovascular risk factors or relevant personal or family medical history and not under chronic medication, went to the emergency department (ED) due to chest pain of 12hours duration accompanied by nausea, sweating and dizziness not relieved by changes in position. On admission to the ED he had residual pain but was hemodynamically stable and physical examination revealed no relevant abnormalities. The electrocardiogram (ECG) revealed sinus rhythm at 72bpm and pathological Q waves in the inferior wall. As he was still symptomatic, he was referred to a center with facilities for primary angioplasty, where he underwent coronary angiography. This showed a left coronary artery without lesions and a dominant right coronary artery with thrombus and spontaneous dissection occupying the entirety of the mid and distal segments, extending to the posterolateral branch and resulting in TIMI 1 flow (Figure 1).
(A and B) Coronary angiography showing the right coronary artery (RCA) with spontaneous dissection from the mid segment and thrombus, resulting in TIMI 1 flow; (C) intravascular ultrasound (IVUS) imaging showing dissection from the posterolateral branch to the mid segment of the RCA with large quantities of thrombus; (D) implantation of three overlapping drug-eluting stents; (E and F) IVUS images showing good stent apposition and expansion and protruding thrombus (arrow).
Since the patient was still in pain during the diagnostic angiography, it was decided to proceed with direct coronary angioplasty assisted by intravascular ultrasound (IVUS) imaging, and three Resolute Onyx™ stents were implanted: one 3.5mm×30mm in the distal segment, one 4mm×38mm proximal to and overlapping the first, and one 4mm×22mm proximal to and overlapping the second. The final result was TIMI 2 flow in the posterior descending and posterolateral arteries. Post-angioplasty control imaging showed good stent apposition and expansion, the presence of intramural hematoma, a degree of protrusion of the in-stent thrombus, and persisting thrombus in the posterolateral artery (Figure 1).
The patient improved to Killip class I, with peak troponin I of 32.5ng/ml and with no recurrence of chest pain during hospital stay and no recorded complex ventricular arrhythmias. The transthoracic echocardiogram showed mildly impaired global systolic function with hypokinesis of the basal and mid segments of the inferior septum and inferior wall.
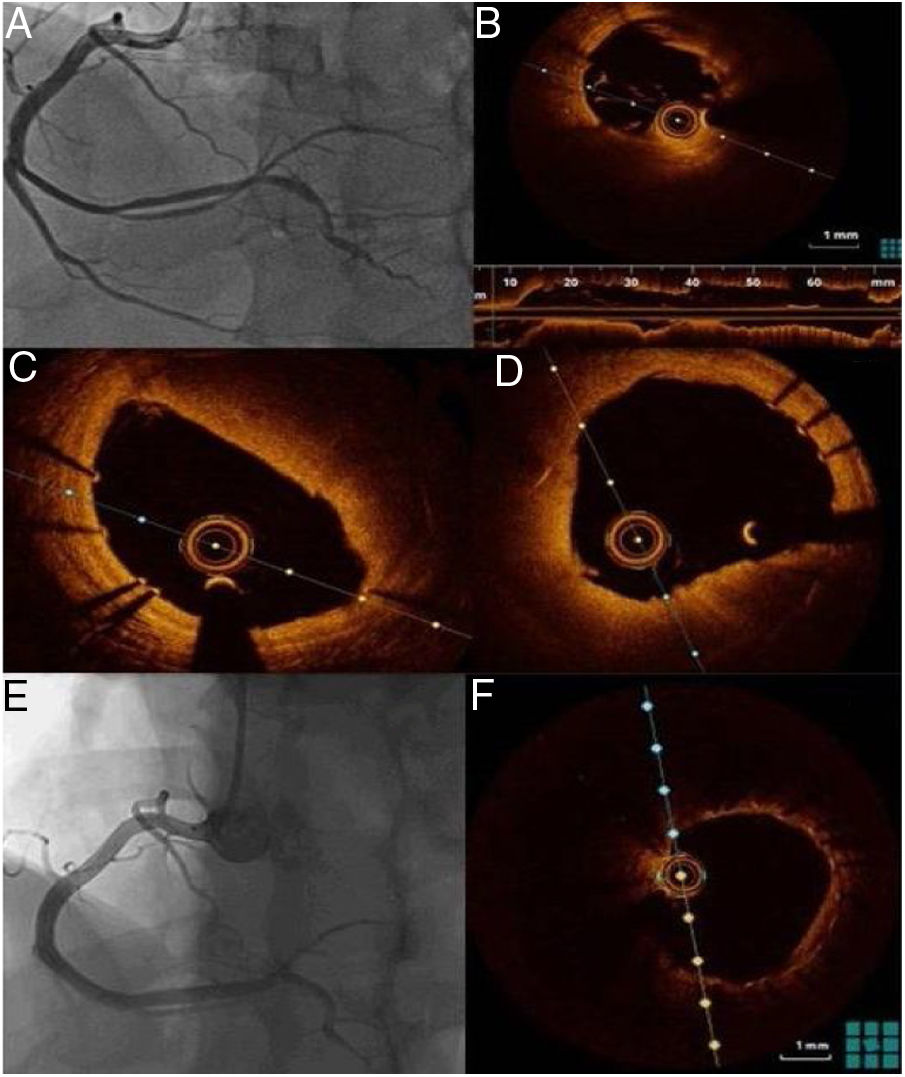
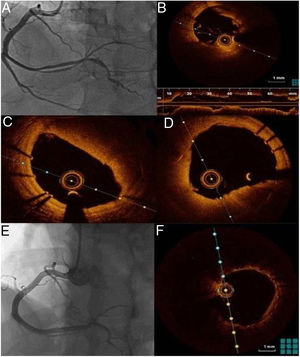
One week after the acute event, coronary angiography was repeated and optical coherence tomography (OCT) was performed, which confirmed good apposition and expansion of the previously implanted stents and the presence of in-stent red thrombus, more clearly visible in the mid-distal part of the vessel, but not significantly obstructing the lumen (Figure 2).
The patient was discharged on day 7 of hospital stay under triple antithrombotic therapy with apixaban 2.5mg twice daily, clopidogrel 75mg once daily and aspirin 100mg once daily; apixaban was discontinued three months after the event. At six months post-event, the patient was asymptomatic with no recurrence of angina, and follow-up angiography with intracoronary imaging showed well-appositioned stents, no red thrombus, and TIMI 3 flow.
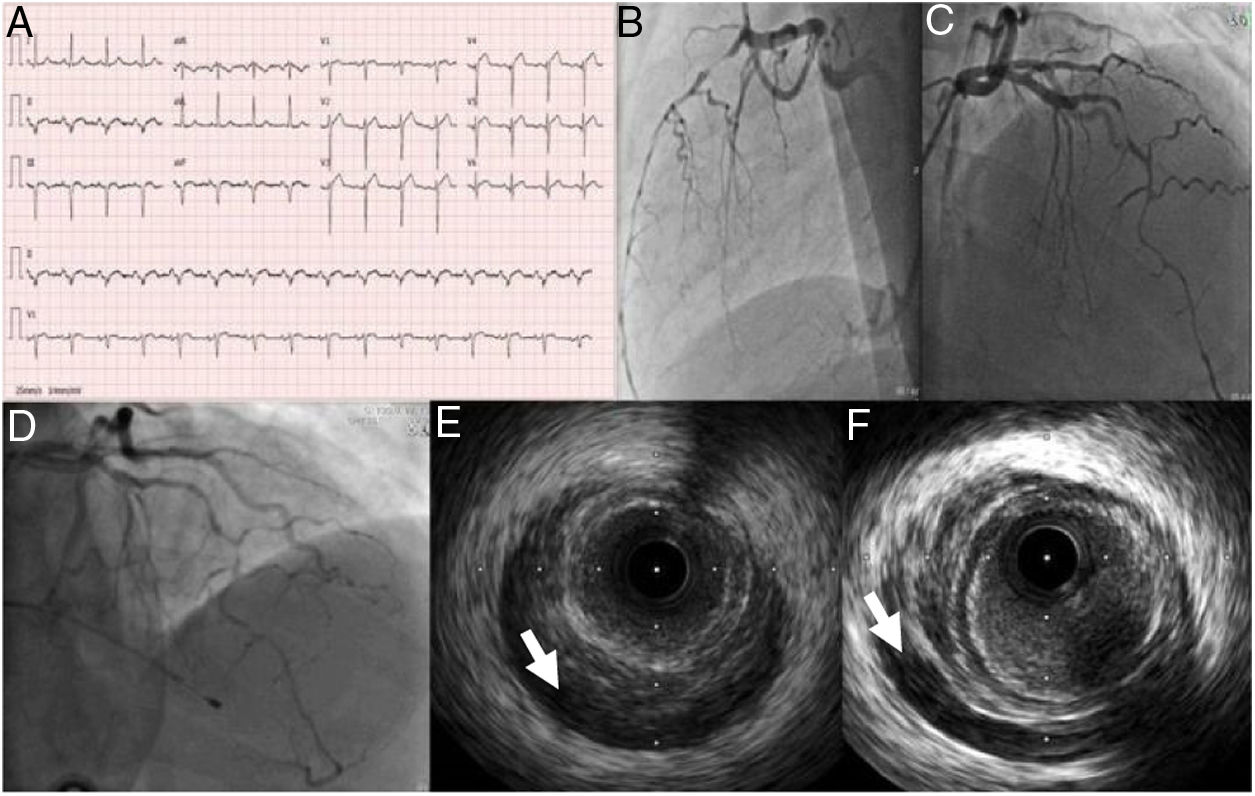
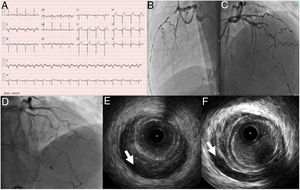
Case 2A 53-year-old man, previously healthy and an active sportsman, with no known cardiovascular risk factors or relevant personal or family medical history and not under chronic medication, went to the emergency department (ED) due to crushing chest pain beginning three days previously and progressively worsening. On admission he was hemodynamically stable with no relevant alterations on physical examination including cardiopulmonary auscultation. The ECG showed sinus rhythm at 93 bpm, ST-segment elevation in the anterior wall and pathological Q waves in the inferior and anterior walls (Figure 3). Urgent cardiac catheterization revealed spontaneous dissection of the left anterior descending artery (LAD), in which, although no flap was visible, intramural hematoma was observed from the mid to the distal segment, resulting in TIMI 2 flow (Figure 3).
(A) Electrocardiogram on admission; (B and C) coronary angiography showing spontaneous dissection of the left anterior descending (LAD) artery occupying all of the mid and distal segments and extending to the posterolateral branch; (D) coronary angiography performed after cardiac arrest 30 hours after admission, revealing apparent progression of the LAD dissection; (E and F) intravascular ultrasound images of an intramural hematoma extending from the proximal LAD (arrow).
Since the patient was hemodynamically and electrically stable and had only residual pain and TIMI 2 flow, a watchful waiting approach was adopted and he was admitted to the coronary care unit for surveillance and monitoring under triple antithrombotic therapy with aspirin, clopidogrel and subcutaneous enoxaparin at therapeutic doses.
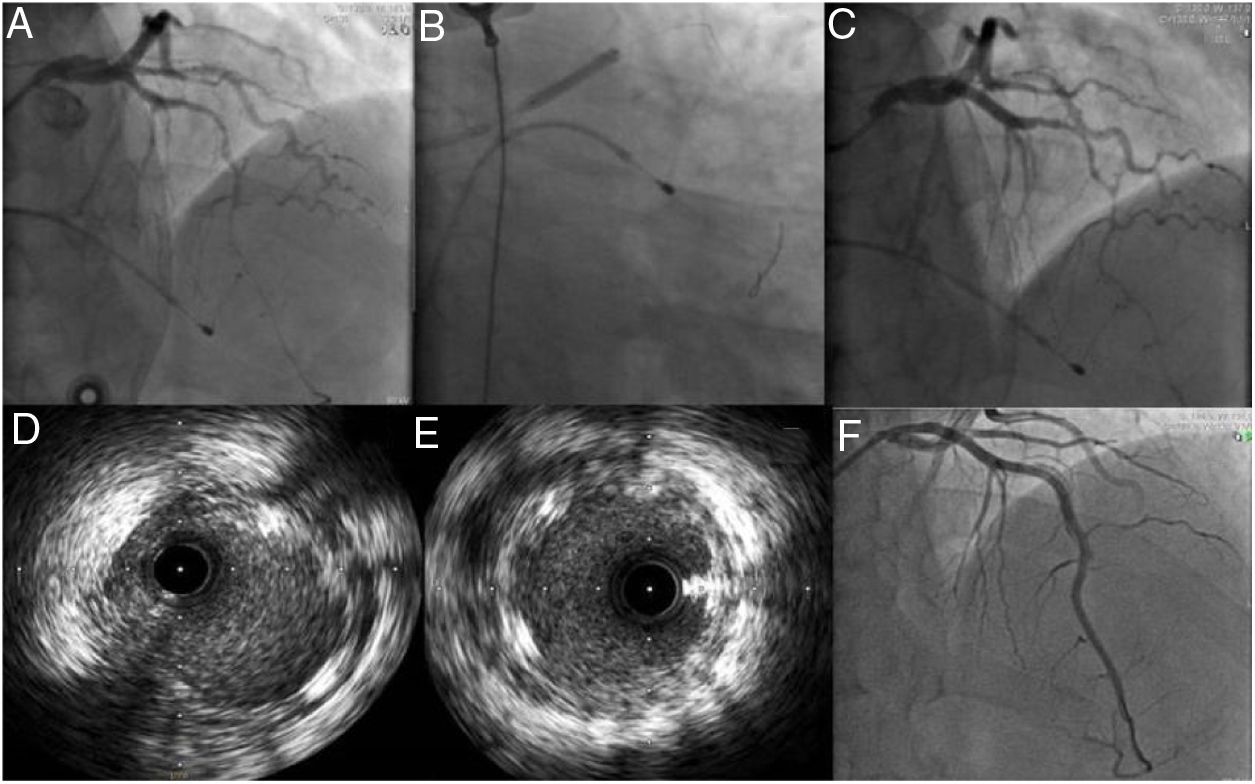
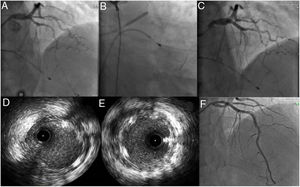
During day 1 of hospital stay, the patient suffered cardiac arrest in asystole preceded by severe bradycardia, from which he recovered after two cycles of advanced life support. It was decided to place a temporary pacemaker and to repeat coronary angiography with IVUS, which confirmed the positioning of the guidewire in the true lumen and revealed apparent progression of the LAD dissection to the proximal third of the artery. Given the patient’s hemodynamic and electrical instability, it was decided to perform angioplasty of the LAD and to place a 3.50mm×23mm Absorb™ bioresorbable vascular scaffold in the proximal segment overdilated with a 4.00mm×20mm non-compliant balloon. Distal flow in the vessel remained at TIMI 2 (Figure 4). Echocardiography performed on day 1 showed moderately impaired global systolic function (left ventricular ejection fraction ∼45%) and akinesis of the apex and apical segments and hypokinesis of the mid segment of the interventricular septum and anterior wall. On day 3, as there had been no further episodes of electrical instability, the temporary pacemaker was removed. Despite hemodynamic, electrical and clinical stability, laboratory tests showed no decrease in myocardial necrosis markers, which varied over the course of hospitalization. Accordingly, after multidisciplinary discussion, on day 6 it was decided to perform angioplasty of the mid and distal segments with predilation with a 2.5mm×30mm balloon followed by placement of two Absorb™ scaffolds, 3mm×28mm and 2.5mm×23mm. As this was an area of myocardial bridging, a drug-eluting stent (2.5mm×38mm XIENCE Alpine™) was implanted overlapping the scaffolds, with a good final result (Figure 4).
(A) Passage of the floppy guidewire to the distal left anterior descending artery; (B and C) implantation of a 3.50mm×23mm AbsorbTM bioresorbable vascular scaffold; (D and E) intravascular ultrasound images after implantation of the Absorb scaffold showing good apposition and expansion, sealing the proximal entry to the dissection; (F) implantation on day 6 of two AbsorbTM scaffolds (3mm×28mm and 2.5mm×23mm) and a XIENCE AlpineTM drug-eluting stent (2.5mm×38mm), with a good final angiographic result.
The patient was discharged on day 9 under triple antithrombotic therapy with apixaban 2.5mg twice daily, clopidogrel 75mg once daily and aspirin 100mg once daily. After one month of follow-up, he remained asymptomatic with no recurrence of angina. Angiographic follow-up with IVUS was scheduled for six months after the acute event.
DiscussionThese two cases illustrate the diagnostic and therapeutic challenges of SCAD.
SCAD is defined as spontaneous separation of the coronary artery wall that is not iatrogenic or related to trauma, creating a false lumen.1
Although its incidence and prevalence have not been properly determined, due to under-diagnosis, SCAD is estimated to account for around 3% of acute coronary syndrome (ACS), with 15% recurrence at two years.1–6
The most commonly affected artery is the LAD. The underlying etiology appears to be multifactorial; it is associated in 72–86% of cases with fibromuscular dysplasia.1
Although SCAD has been historically associated with the peripartum period, this association has been observed less often in recent registries, in which pregnancy-related SCAD accounts for less than 5% of cases. Several chronic systemic inflammatory diseases and connective tissue diseases have been reported to be associated with SCAD, particularly Marfan syndrome and Ehler–Danlos syndrome.7,8
SCAD usually results from a triggering factor in the presence of a genetic and anatomic predisposition. Conditions that increase thoracoabdominal pressure or raise catecholamines can increase cardiocirculatory shear stress, which can trigger the acute event. These triggers include emotional stress, physical activities (especially isometric exercise), drugs, and intense Valsalva-like activities such as childbirth, coughing, or vomiting.9–12
There are various forms of clinical presentation, of which ACS is the most common. Since coronary angiography is the gold standard for patients with ACS, it is the most frequent diagnostic modality in SCAD.1
Intracoronary imaging methods, particularly OCT and IVUS, are important tools in SCAD, providing complementary diagnostic information. The superior image quality of OCT enables better visualization of intimal tears, intraluminal thrombi, the false lumen and intramural hematoma, while IVUS provides a deeper view of the vessel, due to its greater penetration, and can thus give a better picture of the extent of the hematoma. There are also risks involved when performing OCT in such cases, since the dissection can be worsened by contrast injection.13,14
Optimal management of patients with SCAD is not clearly established, since there have been no randomized trials comparing medical therapy with revascularization. However, on the basis of observational studies, clinical outcomes appear to be better with a conservative approach.1
The ideal pharmacological therapy for these patients is the subject of debate.1 On the basis of the evidence on aspirin in ACS and in secondary prevention, together with its low side effect profile, aspirin is generally accepted for acute and long-term SCAD management. Although there are no data on the use of clopidogrel in SCAD patients not treated with stents, dual antiplatelet therapy with aspirin and clopidogrel for between one month and one year may be recommended in order to reduce the prothrombotic state.1,15
The value of the new P2Y12 inhibitors ticagrelor and prasugrel has not been determined. There is also disagreement concerning anticoagulation: on one hand there is a risk of it extending the dissection, while on the other hand it can potentially resolve thrombi formed due to blood pooling in the false lumen and improve true lumen patency.1
Until the results of the SAFER-SCAD randomized trial are available, angiotensin-converting enzyme inhibitors should only be administered to patients with ventricular dysfunction.1,9
A conservative strategy is recommended not only because SCAD commonly resolves with spontaneous angiographic healing but also because most cases of recurrence involve different vessels from the originally affected artery. According to a recent registry, only 17% (six out of 35) patients who underwent revascularization suffered recurrence in the vessel originally involved, and none of the patients treated by a conservative approach (p=0.002).1,16
There is, however, a subgroup of patients for whom percutaneous or surgical revascularization is indicated, including those with recurrent ischemia, hemodynamic or electrical instability, or left main dissection.1,5–9
Percutaneous coronary intervention should be performed cautiously with meticulous techniques, but even then may still result in suboptimal outcomes. The extent of intramural hemotoma is often underestimated by angiography, leading to unforeseen flow loss after stent implantation. In addition, underestimation of the extent of hematoma can result in undersized stents being implanted, increasing the risk of late thrombosis due to stent malapposition following resorption of the hematoma.1,6
Although data on bioresorbable vascular scaffolds in SCAD are scarce, we opted to use them in case 2, in view of the young age of the patient and the fact that the affected vessel had no calcium load. These factors have been described as decisive in the option to use scaffolds rather than drug-eluting stents, with promising long-term results, by Basavarajaiah et al. and Watt et al.17–19
Given the association of SCAD with fibromuscular dysplasia, some authors suggest excluding dissection of the iliac, renal arteries, the great vessels through imaging studies.1
ConclusionSCAD is a therapeutic challenge in which the selected approach should be based on clinical presentation and coronary anatomy. A conservative strategy should be the first option, reserving revascularization for patients with recurrent ischemia, hemodynamic or electrical instability or left main dissection.
Please cite this article as: Martins JL, et al. Disseção coronária espontânea – To stent or not to stent that is the question. Rev Port Cardiol. 2019;38:609.